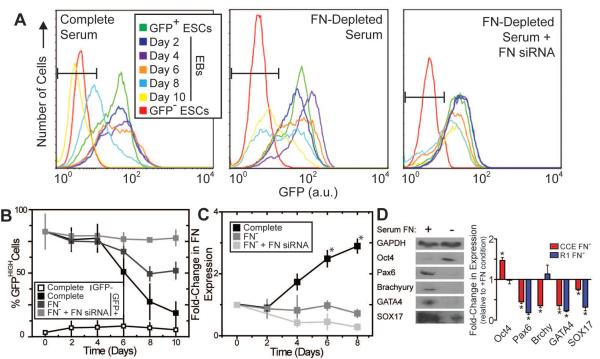
Figure 1. Mouse ESC self-renewal and differentiation is affected by FN.
Mouse ESCs (CCE cell line), expressing GFP under the control of a Nanog promoter, were cultured as embryoid bodies (EBs). (A) EBs, grown in conditions with complete serum or FN-depleted serum ± siRNA for FN, were dissociated into single cells and analyzed by flow cytometry. Mouse ESCs lacking Nanog-GFP were labeled GFP− ESCs (red lines) and used to set a GFPHIGH threshold in B. The starting populations of undifferentiated Nanog-GFP cells were labeled GFP+ ESCs (green lines). (B) The GFPHIGH ESC fraction, determined from the gate in A, as defined by the GFP− ESCs, decreases with time depending on the specific culture conditions. (C) EBs grown in conditions with complete serum or FN-depleted serum (labeled as FN−) ± siRNA for FN were metabolically labeled with [35S] methionine, and the labeled FN produced during labeling was analyzed by SDS-PAGE and phosphorimaging (images not shown). Data is normalized to the FN produced by undifferentiated ESCs at day 0. (D) Mouse ESCs (CCE and R1) were cultured as EBs for six days in medium with complete (+) or FN-depleted serum (−). Cells were lysed and equal amounts of protein were separated by SDS-PAGE and blotted with Oct4 (self-renewal), Pax6 (neural progenitor), Brachyury (mesoderm), GATA4 (definitive endoderm), SOX17 (definitive endoderm) and GAPDH (loading control) antibodies (left). The fold-change in protein expression of the FN− culture relative to the FN+ culture was quantified (mean ± standard error from 3 independent samples) (right). (A–C) * p < 0.05 as determined by ANOVA. (D) * p<0.05 as determined by a one-sample t-test comparing the sample to a theoretical mean of 1.0.