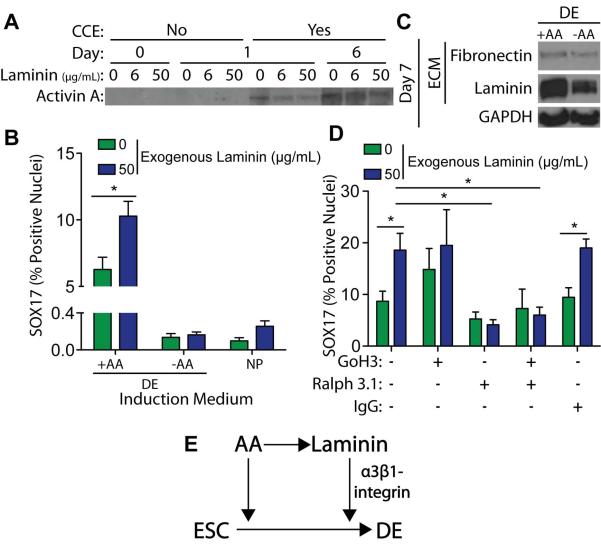
Figure 7. Laminin Enhances DE Induction through α3-integrin mediated signaling.
(A) Decellularized 3T3-derived ECM, grown with the indicated concentration of exogenous mouse laminin-111, was incubated in DE induction medium, containing 100 ng/mL Activin A, either in the presence (Yes) or absence (No) of mouse ESCs (CCE cell line) for the indicated number of days. The DOC-insoluble matrix was separated by SDS-PAGE and blotted for bound Activin A. (B) 3T3-derived ECMs, grown with the indicated concentration of exogenous laminin-111, were decellularized and reseeded for 6 days with naïve mouse ESCs (R1 cell line) in either neural progenitor (NP) or definitive endoderm (DE) induction, with or without 100 ng/mL exogenous Activin A. Immunofluorescent images of SOX17 and DAPI stain nuclei were taken and the percentage of SOX17 positive nuclei was quantified (>103 nuclei/group) (mean ± standard error). (C) Mouse ESCs (R1 cell line) were grown for 7 days on a gelatin-coated substrate in DE induction medium, with or without 100 ng/mL Activin A. A DOC-solubility assay was used to isolate the DOC-soluble cell lysis fraction from the DOC-insoluble extracellular matrix. Both fractions were separated by SDS-PAGE and blotted for GAPDH and extracellular matrix proteins, respectively. (D) 3T3-derived ECMs, grown with the indicated concentration of exogenous laminin, were decellularized and reseeded for 6 days with naïve mouse ESCs (R1 cell line) in DE medium containing Activin A and the indicated integrin function-blocking antibodies. Immunofluorescent images of SOX17 and DAPI stained nuclei were taken and the percentage of SOX17 positive nuclei was quantified (mean ± standard error). (E) Schematic of proposed feed-forward mechanism in which Activin A enhances endogenous laminin expression and laminin-mediated alpha-3 integrin signaling enhances the DE induction efficiency of Activin A. * p < 0.05 as determined by ANOVA.