Abstract
Background
Ethanol (EtOH) decreases muscle protein synthesis, and this is associated with reduced mTORC1 and increased mTORC2 activities. In contrast, phospholipase D (PLD) and its metabolite phosphatidic acid (PA) positively regulate mTORC1 signaling, whereas their role in mTORC2 function is less well defined. Herein, we examine the role that PLD and PA play in EtOH-mediated mTOR signaling.
Methods
C2C12 myoblasts were incubated with EtOH for 18–24 h. For PA experiments, cells were pre-treated with the drug for 25 min followed by 50 min incubation with PA in the presence or absence of EtOH. The phosphorylation state of various proteins was assessed by immunoblotting. Protein-protein interactions were determined by immunoprecipitation and immunoblotting. PLD activity was measured using the Amplex red phospholipase D assay kit. PA concentrations were determined with a total PA assay kit.
Results
PA levels and PLD activity increased in C2C12 myocytes exposed to EtOH (100 mM). Increased PLD activity was blocked by inhibitors of AMPK (compound C) and PI3K (wortmannin). Likewise, suppression of PLD activity with CAY10594 prevented EtOH-induced Akt (S473) phosphorylation. PLD inhibition also enhanced the binding of Rictor to mSin1 and the negative regulatory proteins Deptor and 14-3-3. Addition of PA to myocytes decreased Akt phosphorylation, but changes in mTORC2 activity were not associated with altered binding of complex members and 14-3-3. PA increased S6K1 phosphorylation, with the associated increase in mTORC1 activity being regulated by reduced phosphorylation of AMPKα (T172) and its target TSC2 (S1387). This resulted in increased Rheb and RagA/ RagC GTPase interactions with mTOR, as well as suppression of mTORC2.
Conclusions
EtOH-induced increases in PLD activity and PA may partially counterbalance the adverse effects of this agent. EtOH and PA regulate mTORC1 via a PI3K/AMPK/TSC2/PLD signaling cascade. PA stimulates mTORC1 function and suppresses activation of mTORC2 as part of an mTORC1/2 feedback loop.
Keywords: Ethanol, phosphatidic acid, 14-3-3 proteins, Rag GTPases, protein synthesis
INTRODUCTION
The mammalian target of rapamycin (mTOR) is a Ser/Thr protein kinase that integrates cues from a number of signals (cellular stresses, nutrients, growth factors and hormones) to regulate cell growth, metabolism and survival (Sarbassov et al., 2005; Wullschleger et al., 2006). mTOR exerts its effect as part of two biochemically and functionally distinct protein complexes termed mTOR complex (mTORC)1 and mTORC2 (Loewith et al., 2002). In addition to mTOR, mLST8/GβL and Deptor (DEP- domain-containing mTOR-interacting protein) are members of both mTORC1 and mTORC2. Raptor (regulatory associated protein of mTOR) and PRAS40 (proline-rich Akt substrate 40 kDa) are unique members of mTORC1, whereas Rictor (rapamycin-insensitive companion of mTOR), mSin1 (mammalian stress-activated protein kinase (SAPK)-interacting protein) and Protor/PRR5 (protein observed with rictor/proline-rich repeat protein 5) are distinct components of mTORC2 (Bai and Jiang, 2010; Dunlop and Tee, 2009).
mTOR inputs from anabolic and catabolic stimuli are mediated via different signals including the PI3K/Akt and AMPK (AMP-activated protein kinase) pathways (Bibollet-Bahena and Almazan, 2009; Faghiri and Bazan, 2010; Memmott and Dennis, 2009; Mounier et al., 2011). Increased mTORC1 activity following stimulation of the PI3K/Akt pathway occurs in concert with modulation of the inhibitory effects of PRAS40 and the tumor suppressor tuberous sclerosis protein complex (TSC) 2 (Inoki et al., 2002; Sancak et al., 2007). In contrast, under stress conditions, mTORC1 is influenced by AMPK/TSC2 signaling events. AMPK is a major nutrient and cell energy sensing protein activated under unfavorable conditions (e.g., EtOH, hypoxia, glucose and serum deprivation) (Hong-Brown et al., 2010; Inoki et al., 2003b). As such, this kinase downregulates energetically demanding processes such as protein synthesis and cell growth. The activation of AMPK leads to increased phosphorylation of TSC2, a protein that serves as a GTPase activator protein (GAP) for Rheb (Ras homolog enriched in brain) (Inoki et al., 2003a). Whereas Akt mediated phosphorylation of TSC2 (T1462 and S939) is inhibitory, phosphorylation of TSC2 at S1345 and T1227 by AMPK stimulates the negative regulatory function of this protein towards Rheb. Elevated TSC2 phosphorylation at S1387 decreases the association of Rheb and Rag with mTOR (Hong-Brown et al., 2012a) This, in turn, inhibits the ability of mTORC1 to regulate the downstream proteins 4E-BP1 and S6K1, which play a central role in regulating protein synthesis.
Several lines of evidence suggest that growth factor-stimulated mTORC1 activity suppresses mTORC2 function via a negative feedback loop, thereby implicating interplay between these two multimeric protein complexes (Dibble et al., 2009; Foster and Fingar, 2010; Treins et al., 2010). In contrast, reduction of mTORC1 activity by EtOH is associated with a reciprocal activation of mTORC2. For example, diminishing phosphorylation of S6K1 reduces Rictor phosphorylation, as well as its interaction with 14-3-3 proteins and other mTORC2 components. This dissociation stimulates mTORC2 activity and enhances Akt S473 phosphorylation. Together, these events function as part of a feedback loop between mTORC1 and mTORC2, whereby suppression of mTORC1 activates mTORC2 (Hong-Brown et al., 2011; 2012b).
mTORC1 and mTORC2 functions can be controlled via phospholipase D (PLD) signaling, and this occurs following mitogenic and mechanical stimuli as well as under stress conditions (Fang et al., 2001; Hornberger et al., 2006; Toschi et al., 2009). PLD is a membrane-bound enzyme which is important in receptor-mediated signaling transduction. In mammalian cells, PLD exits in two distinct isoforms named PLD1 and PLD2. PLD catalyzes the hydrolysis of phosphatidylcholine (PC) leading to the generation of choline and the lipid second messenger phosphatidic acid (PA). PLD is activated by mitogenic factors (e.g. serum, amino acids, glucose, epidermal growth factor, and electrical-induced muscle contraction) (Fujita et al., 2008; O'Neil et al., 2009; Xu et al., 2011). Paradoxically, stimulation of PLD has also been observed in various cells types in response to stressful insults, such as serum withdrawal, glucose deprivation and exposure to endotoxin (Kim et al., 2010; Lim et al., 2003; Zheng et al., 2006). Thus, both inhibitors and stimulators of growth can, under appropriate conditions, activate PLD. In contrast, PLD signaling is inhibited by primary alcohols such as EtOH and 1-butanol (Fujita et al., 2008; Kotter and Klein, 1999). Therefore, it is possible the effect of EtOH on mTORC1 and mTORC2 is mediated by PLD and PA.
The signal transduction pathway(s) linking PLD to mTORC1 remain enigmatic. A recent study showed that amino acids and glucose stimulated PLD and mTORC1, and this was regulated by the class III PI3K hVps34 (Xu et al., 2011). In contrast, under stress situations, PLD activity was mediated by AMPK and the ERK pathway (Kim et al., 2010). Akt has also been shown to stimulate PLD activity, with this occurring via activation of the ERK pathway (Patel et al., 2010). The mechanism through which PA stimulates mTOR is also controversial. For example, PA has been shown to bind to mTOR and activate this protein (Fang et al., 2001). Conversely, PA can activate mTOR indirectly via ERK-dependent signaling (Winter et al., 2010). Whereas mTORC1 signals to S6K1 following PA binding, it has been reported that PLD 2- derived PA can directly interact with S6K1 to stimulate this kinase in a mTOR-independent manner (Lehman et al., 2007).
PLD activity appears to link a number of disparate factors that play roles in regulating mTORC1 and mTORC2 (Fang et al., 2001; Hornberger et al., 2006; Toschi et al., 2009; Xu et al., 2011). Therefore, the aim of the present study was to examine whether the effects of EtOH on mTOR signaling are mediated by PLD and PA. The mechanism by which PLD and PA regulate mTOR complexes as well as the association of 14-3-3 with various mTORC1 and mTORC2 components was also examined. EtOH increased PLD activity and PA contents in myocytes. This increase was dependent on AMPK/TSC2/Rheb and PI3K signaling, as pharmacological inhibitors of these pathways blocked PLD induction. The addition of PA to cells increased mTORC1 activity, but it diminished the alcohol-induced increase in mTORC2 signaling. We propose that increases in PLD activity and PA partially counterbalance the adverse effects of EtOH in myocytes.
MATERIALS AND METHODS
Materials
Ethanol was purchased from Fisher Scientific Co. (Springfield, NJ). Antibodies against Rictor and mSin1 were from Bethyl Laboratories, Inc. (Montgomery, TX). 14-3-3 Ѳ and Rheb antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against phosphorylated (p-) mTOR (S2448), p-Rictor (T1135), p-Akt (S473), p-S6K1 (T389), p-S6 ribosomal protein (S235/236), p-4EBP1 (T37/46), p-TSC2 (S1387) and p-AMPKα (T172) were purchased from Cell Signaling Technology (Beverly, MA). Antibodies to RagA, RagC, mTOR, Raptor, PLD and β-actin were obtained from the same source, with the latter serving as a control for equal protein loading of samples. The PLD inhibitor CAY 10594 and a PA assay kit were purchased from Cayman Chemical Company (Ann Arbor, MI). PA (1,2-Dioctanoyl-sn-Glycero-3-phophate; monosodium salt) was from Avanti Polar Lipids, Inc (Alabaster, AL). The PLD assay kit was obtained from Life Technologies Corporation (Carlsbad, CA). The AMPK inhibitor compound C was from EMD Millipore Corporation (Chicago, IL). Wortmannin, phenylmethanesulfonyl fluoride (PMSF), protease, phosphatase I and II inhibitor cocktails were from Sigma (St. Louis, MO). Cell culture media and fetal bovine serum (FBS) were from Mediatech, Inc (Manassas, VA).
Cell culture
C2C12 mouse myoblasts (American Type Culture Collection; Manassas, VA) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS, penicillin (100 U/ml), streptomycin (100 µg/ml) and amphotericin (25 µg/ml) at 37°C in 5% CO2. Cells were plated on 6-well or 10 cm dishes and grown to confluence. All studies were conducted using cells at the early passage (p3-p7) of the myoblast stage. Upon confluence, cells were switched to 1.5 % fetal bovine serum (FBS) media in the presence or absence of 100 mM EtOH for 18–24 h as previously described (Hong-Brown et al., 2007) The presence of serum was necessary because the survival of C2C12 myocytes is decreased when cells are cultured for extended periods in serum-free media (SFM). Likewise, this concentration of EtOH was used because it produces an optimal effect without being cytotoxic to myocytes (Hong-Brown et al., 2006). During the incubation, plates were sealed with parafilm to minimize the evaporation of EtOH.
Drug treatments
PA in powder form was dissolved in phosphate buffered saline (PBS), whereas PLD inhibitor, compound C and wortmannin were dissolved in DMSO. For PA experiments, cells were pre-treated with the drug in SFM for 25 min. Cells were then given an additional 50 min incubation with PA in the presence or absence of EtOH. For studies using the PLD inhibitor, compound C and wortmannin, cells were pre-treated as noted in the Figure Legends. The concentration and incubation times for reagents were chosen based on dose- and time-response curves derived from our preliminary studies and the literature (Hong-Brown et al., 2010; Kotter and Klein, 1999; O'Neil et al., 2009). Total protein was determined using the bicinchoninic acid reagent (BCA) protein assay kit with bovine serum albumin as a standard (Pierce, Rockford, IL). The results were normalized with total protein and compared with the control group. Data were expressed as a percentage of the control value.
PLD activity and total PA concentration
PLD activity was measured using the Amplex red phospholipase D assay kit (A122189) according to the manufacturer’s protocol. PLD activity was monitored indirectly using 10-acetyl-3,7-dihydrophenoxazine (Amplex Red reagent), a sensitive fluorogenic probe for H2O2. The PA concentration of cells was determined using a Cayman’s total PA assay kit (700240) which provides a fluorescence-based method for measuring total PA in cellular lipids.
Immunoprecipitation and immunoblot analysis
C2C12 myocytes, cultured in either 10 cm or 6-well plates, were incubated in the presence of EtOH and/or various reagents (PA, PLD inhibitor). Thereafter, cells were rinsed once with PBS and lysed in ice-cold 0.3% CHAPS (3-[(3-cholamindopropyl) dimethylammonio]-1-propanesulfonate) buffer containing PMSF and a cocktail of protease and phosphatase inhibitors. Soluble fractions of cell extracts were isolated by centrifugation at 14,000 rpm for 5 min at 4°C. For immunoprecipitation (IP), a 1:50 dilution of primary antibody (Ab) was added to equal amounts of protein (300–500 µg) from cell lysates and rotated overnight at 4°C. A total of 40 µl of a 50% slurry of protein A sepharose was then added for an additional 1 h. Immunoprecipitates were washed 3 times with lysis buffer and the precipitated proteins were denatured by the addition of 2x Laemmli sample buffer (LSB). Equal amounts of protein from cell lysates were electrophoresed on denaturing polyacrylamide gels and transferred to nitrocellulose membranes. The resulting blots were blocked with 5% nonfat dry milk and incubated with the antibodies of interest (1:1000). Unbound primary antibody was removed by washing with TBS containing 0.05% Tween-20 (ICI Americas, Inc, Wilmington, DE) and blots were incubated with anti-rabbit immunoglobulin (1:3000) conjugated with horseradish peroxidase (HRP). On occasions where the denatured and reduced rabbit IgG light or heavy chains obscured protein bands of similar molecular weights, mouse anti-rabbit IgG (conformation specific L27A9) mAb (Cell Signaling) was added as a bridging antibody prior to incubation with an anti-mouse IgG, HRP-linked secondary antibody (Cell Signaling). Blots were developed with an enhanced Amersham chemiluminescent detection system (GE Healthcare, Buckinghamshire, UK) and exposed to MiDSci X-ray blue film (Cole Parmer, Chicago, IL). The film was scanned (Epson perfection V500 Photo) and analyzed with Scion image version 3b software (NIH, Bethesda, MD).
Statistical analysis
For experimental protocols with more than two groups, statistical significance was determined using one-way ANOVA followed by the Dunnett’s test to compare all data to the appropriate control group. For experiments with only two groups, an unpaired Student’s t-test was performed. Data are presented as mean ± SE. A value of P < 0.05 was considered statistically significant.
RESULTS
Ethanol increases PA content and PLD activity
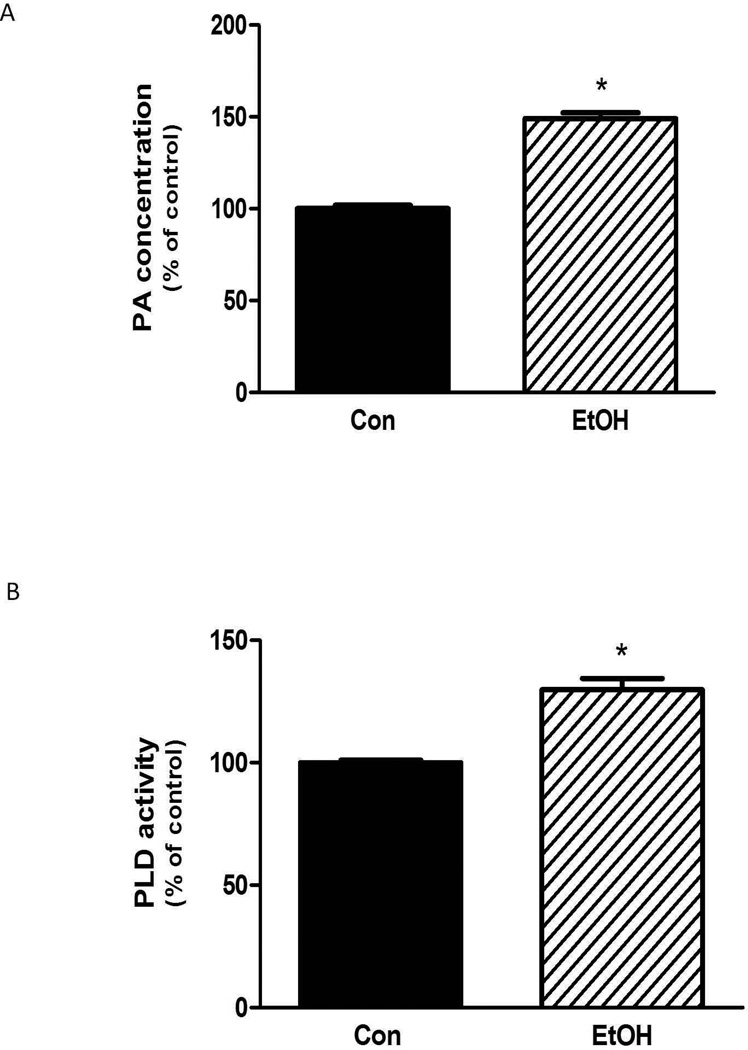
Previous studies have reported that EtOH decreased PA levels in astrocytes and neutrophils (Kotter and Klein, 1999; Tou and Gill, 2005). However, the effects of EtOH on muscle cells have not been examined. In the present study, incubation of C2C12 myocytes with 100 mM EtOH significantly increased the PA level (∼ 50 %) relative to controls (Fig. 1A). Alterations in the concentration of PA are mediated by the activity of PLD, whereas PLD activity is regulated by various stressors and mitogenic stimuli. As shown in Figure 1B, incubation of myocytes with EtOH increased PLD activity by ∼ 30%.
Fig. 1.
EtOH increases PA content and PLD activity in myocytes. C2C12 myocytes were incubated overnight in the presence or absence of 100 mM EtOH. Cells were collected and total PA concentration (A) or phospholipase D activity (B) was determined as described in the “Materials and Methods.” Results were normalized with total protein and expressed as a percentage of control levels. Each bar represents the mean ± SE of 4 independent experiments consisting of 3–5 replicate samples per experiment. P< 0.05 versus control values.
Effects of PLD inhibition on mTORC1 and mTORC2 activity
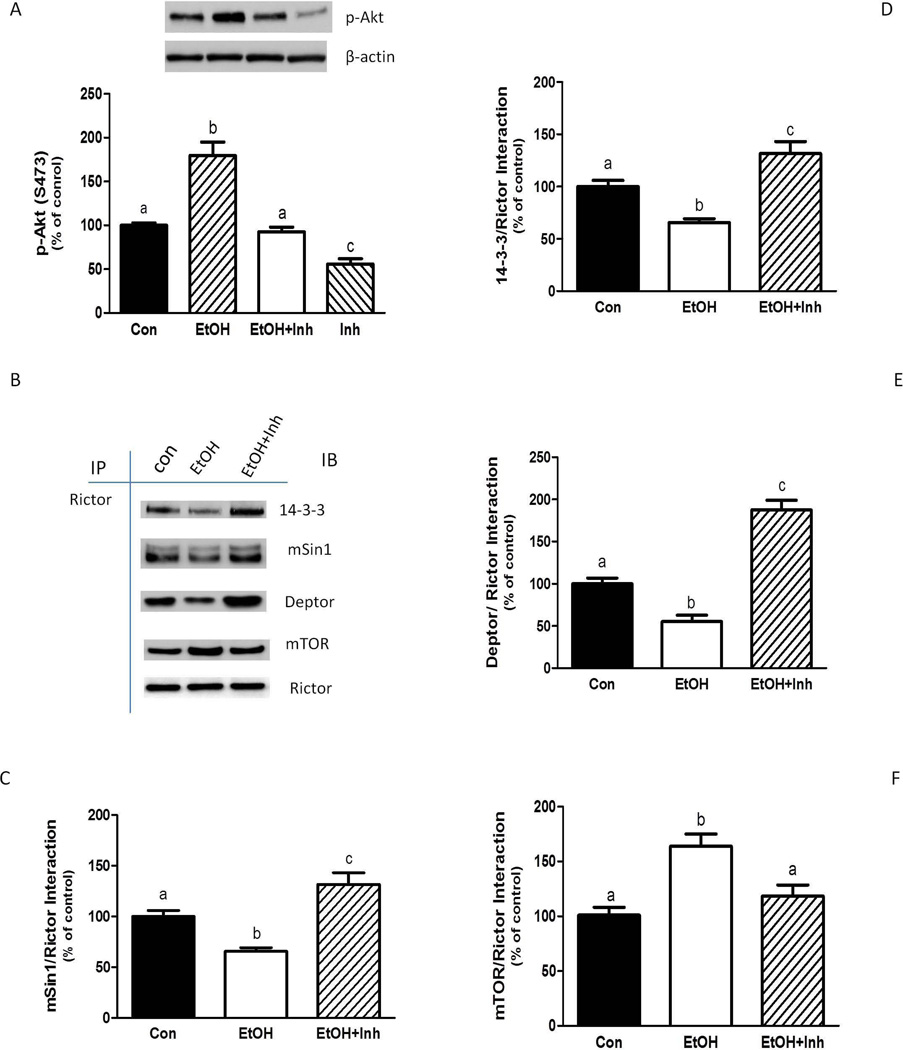
Next, we examined whether PLD is involved in the formation and activation of mTOR complexes, both in the presence and absence of EtOH. The S473-phosphorylation of Akt has been used as an indicator of mTORC2 activity (Hong-Brown et al., 2011). EtOH increased the phosphorylation of Akt at S473 (Fig. 2A), in agreement with our previous findings (Hong-Brown et al., 2010; 2011). In contrast, treatment of myocytes with a PLD inhibitor prevented the induction of Akt phosphorylation by EtOH.
Fig. 2.
The EtOH-induced increase in mTORC2 function is dependent on PLD activity. C2C12 myocytes were incubated in the presence or absence of 100 mM EtOH. The cells were then shifted to SFM with or without 10 µM of PLD inhibitor (Inh). After 20 min., EtOH was added back to the media for another 50 min in the presence or absence of the PLD inhibitor. Equal amounts of protein from cell lysates were analyzed via Western blotting using an antibody against the S473-phosphorylated form of Akt (A). For these experiments, actin was used as a loading control. (B) Rictor was immunoprecipitated from equal amounts of cell extracts and then immunoblotted with antibodies as indicated. Results were quantified as bar graphs (C–F) and normalized with immunoprecipitated (IP) Rictor that was assessed by immunoblotting. The data shown are means ± SE of 3–4 independent experiments consisting of 3–5 replicate samples per experiment. Groups with different letters are significantly different from one another (P< 0.05). Groups with the same letters are not significantly different.
EtOH-induced changes in mTORC2 activity are associated with alterations in protein-protein interactions which mediate downstream signaling events (Hong-Brown et al., 2011). Hence, the association of Rictor with various mTORC2 components and the negative regulatory protein 14-3-3 Ѳ was assessed. EtOH decreased the binding of Rictor with mSin1 (Fig. 2B and 2C). The interaction of Rictor with the negative regulators 14-3-3 Ѳ and Deptor was also reduced (Fig. 2B, 2D and 2E). In contrast, EtOH increased the Rictor and mTOR association (Fig. 2B and 2F). In each case, the addition of the PLD inhibitor ameliorated the effect of EtOH. For example, suppression of PLD activity enhanced the association of mSin1 and Rictor, even in the presence of EtOH (Fig. 2B and 2C). Likewise, the inhibitor increased the binding of Rictor with 14-3-3 Ѳ (Fig. 2B and 2D) as well as Deptor (Fig. 2B and 2E). In contrast, the PLD inhibitor blocked the EtOH-induced increase in the Rictor and mTOR interaction (Fig. 2B and 2F).
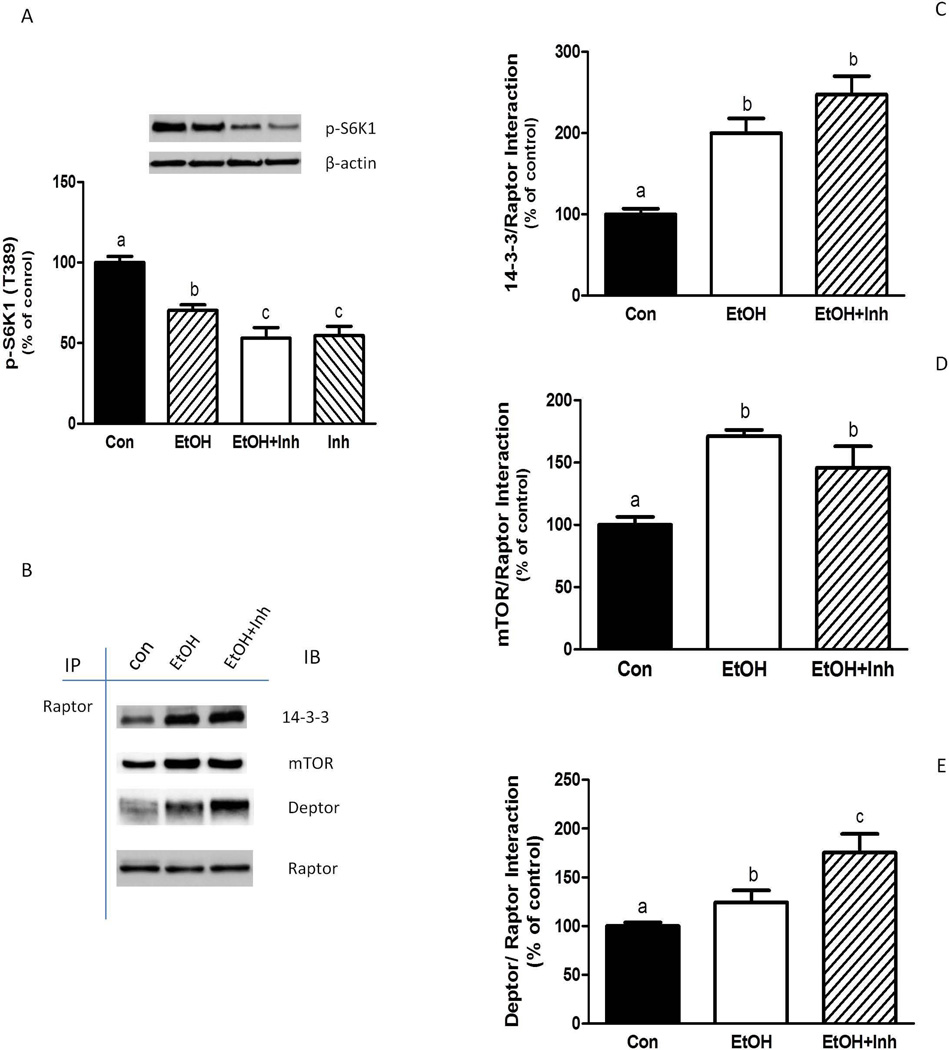
We next examined the effect of PLD inhibition on mTORC1 activity (Fig. 3A). In general, the PLD inhibition produced effects similar to those observed following EtOH exposure. For example, reduced phosphorylation (T389) of S6K1 was observed when cells were incubated with EtOH, the PLD inhibitor, or a combination of both. This decrease in mTORC1 activity was associated with increased interaction of Raptor with 14-3-3 Ѳ, mTOR, and Deptor (Fig. 3B–3E). The combined effects of EtOH and the PLD inhibitor were additive in enhancing the Deptor and Raptor interaction.
Fig. 3.
EtOH-induced suppression of mTORC1 function is not dependent on PLD. C2C12 myocytes were incubated in the presence or absence of EtOH as described in Fig.2. Equal amounts of protein from cell lysates were analyzed via Western blotting using an antibody against the T389-phosphorylated form of S6K1 (A). (B) Raptor was immunoprecipitated from equal amounts of cell extract and then immunoblotted with the indicated antibodies. Results were quantified as bar graphs (C–E) and normalized with immunoprecipitated (IP) Raptor that was assessed by immunoblotting. The data shown are mean ± SE of 4 independent experiments consisting of 4–5 replicate samples per experiment. Groups with different letters are significantly different from one another (P< 0.05). Groups with the same letter are not significantly different.
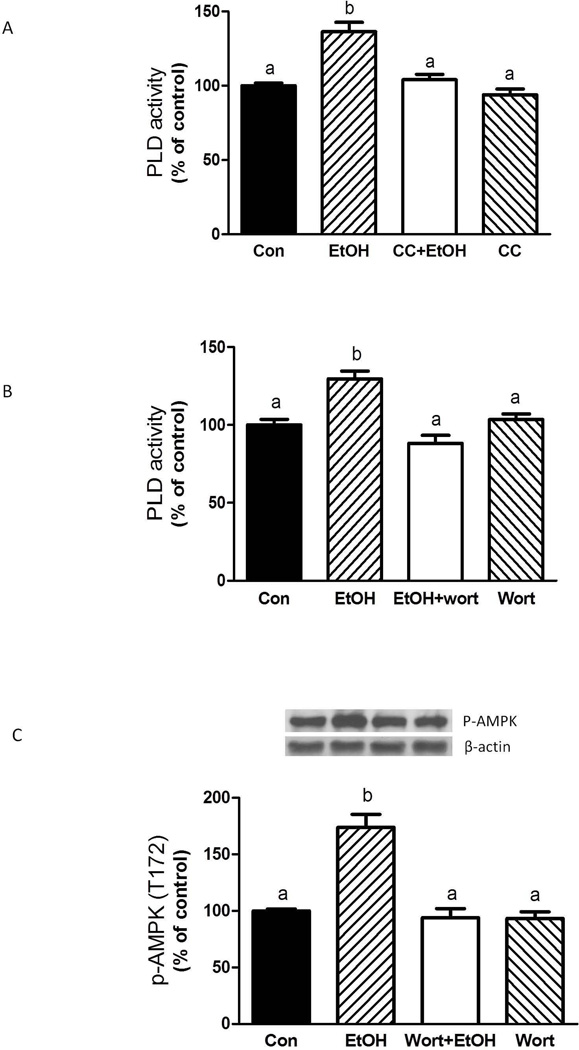
AMPK stimulates PLD activity
EtOH increases both AMPK (Hong-Brown et al., 2007; Hong-Brown et al., 2012a) and PLD activity (Fig. 1B). Therefore, to characterize the connection between these events, myocytes were treated with EtOH and the AMPK inhibitor compound C (CC). Figure 4A shows that incubation of myocytes with CC suppressed the EtOH-induced increase in PLD activity, suggesting a role for AMPK in mediating the effects of EtOH on PLD function. Another potential modulator of EtOH-related PLD signaling is PI3K. Incubation of myocytes with the PI3K inhibitor wortmannin prevented the increase in PLD activity produced by EtOH (Fig. 4B). Wortmannin also inhibited the EtOH-induced increase in phosphorylation of AMPK (Fig. 4C). Collectively, these results demonstrate a link between the PI3K, AMPK and PLD signaling cascades.
Fig. 4.
EtOH-induced PLD activity is mediated by AMPK and PI3 kinase pathways. C2C12 myocytes were incubated overnight in the presence or absence of 100 mM EtOH. The cells were then pre-incubated for 1 h in SFM with either the AMPK inhibitor compound C (CC, 20 µM) or the PI3 kinase inhibitor wortmannin (wort, 100 nM). Thereafter, EtOH was added to the media for an additional 50 min in the presence or absence of inhibitors. Cells were collected and phospholipase D activity was determined (A–B) as described in “Materials and Methods.” (C) Equal amounts of protein from cell lysates were analyzed via Western blotting using an antibody against the phosphorylated form of AMPKα. Results were normalized with total protein and expressed as percentage of control levels. Each bar represents the mean ± SE of 3–4 independent experiments consisting of 3–5 replicate samples per experiment. Groups with different letters are significantly different from one another (P< 0.05). Groups with the same letters are not significantly different.
PA affects mTORC1 and mTORC2 function
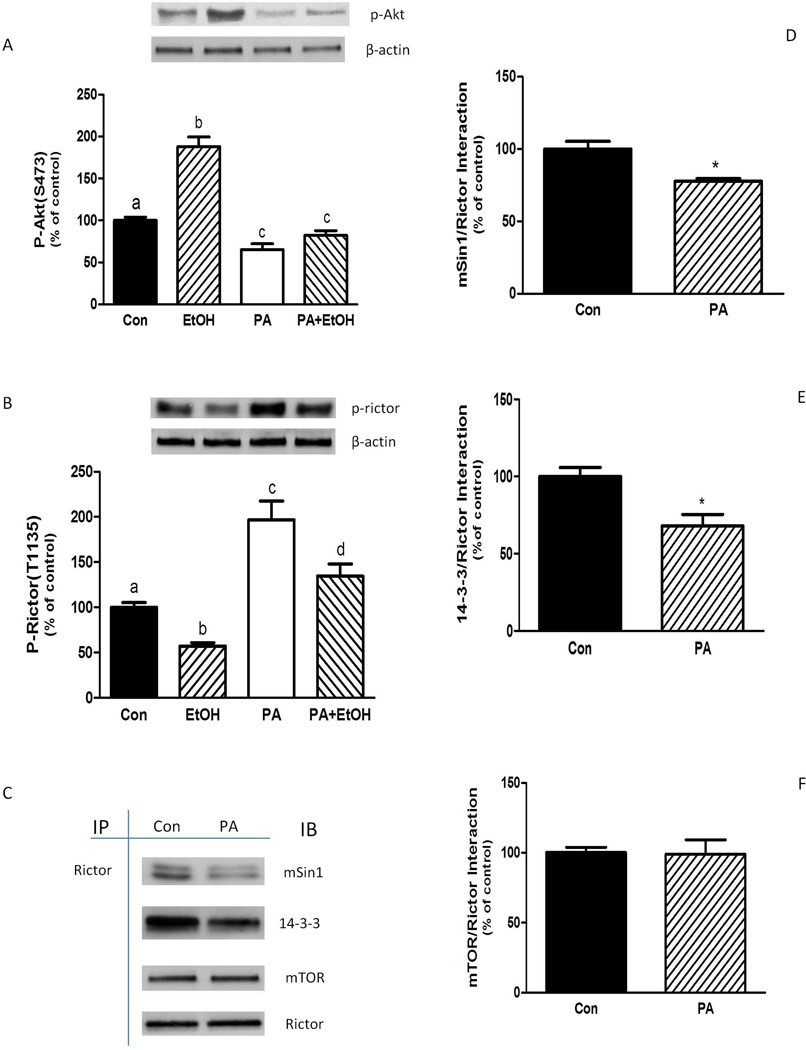
We next investigated the effects of the PLD metabolite PA on mTORC2 activity and assembly. The addition of PA to myocytes decreased S473-phosphorylation of Akt (Fig. 5A), suggesting a decrease in mTORC2 activity. Along these lines, PA also blocked the stimulatory effect of EtOH on Akt phosphorylation. PA increased Rictor phosphorylation, with levels remaining above controls in cells exposed to a combination of EtOH and PA (Fig. 5B). PA also affected the protein-protein interaction of this complex. As illustrated in Figure 5C–5E, there was a decreased interaction of Rictor with mSin1 and 14-3-3 Ѳ. A similar observation was made for Rictor and Deptor binding (data not shown). However, PA did not affect mTOR and Rictor association (Fig. 5C and 5F).
Fig. 5.
PA alters Akt and Rictor phosphorylation, as well as mTORC2 protein-protein interactions. C2C12 myocytes were incubated overnight in the presence or absence of 100 mM EtOH. For PA treatment, cells were incubated in SFM with PA (30 µM) for 75 min. For combination experiments, EtOH pre-treated cells were incubated in SFM with PA (30 µM) for 25 min. EtOH was then added to the media for another 50 min in the continued presence of PA. Equal amounts of protein from cell lysates were analyzed via Western blotting using antibodies against the phosphorylated forms of Akt (A) and Rictor (B). (C) Rictor was immunoprecipitated from equal amounts of cell extract and then immunoblotted with antibodies as indicated. Results were quantified as bar graphs (D–F) and normalized with immunoprecipitated (IP) Rictor that was assessed by immunoblotting. The data shown are mean ± SE of 4 independent experiments consisting of 3–5 replicate samples per experiment. Groups with different letters are significantly different from one another (P< 0.05). Groups with the same letters are not significantly different. *P< 0.05 versus the control values.
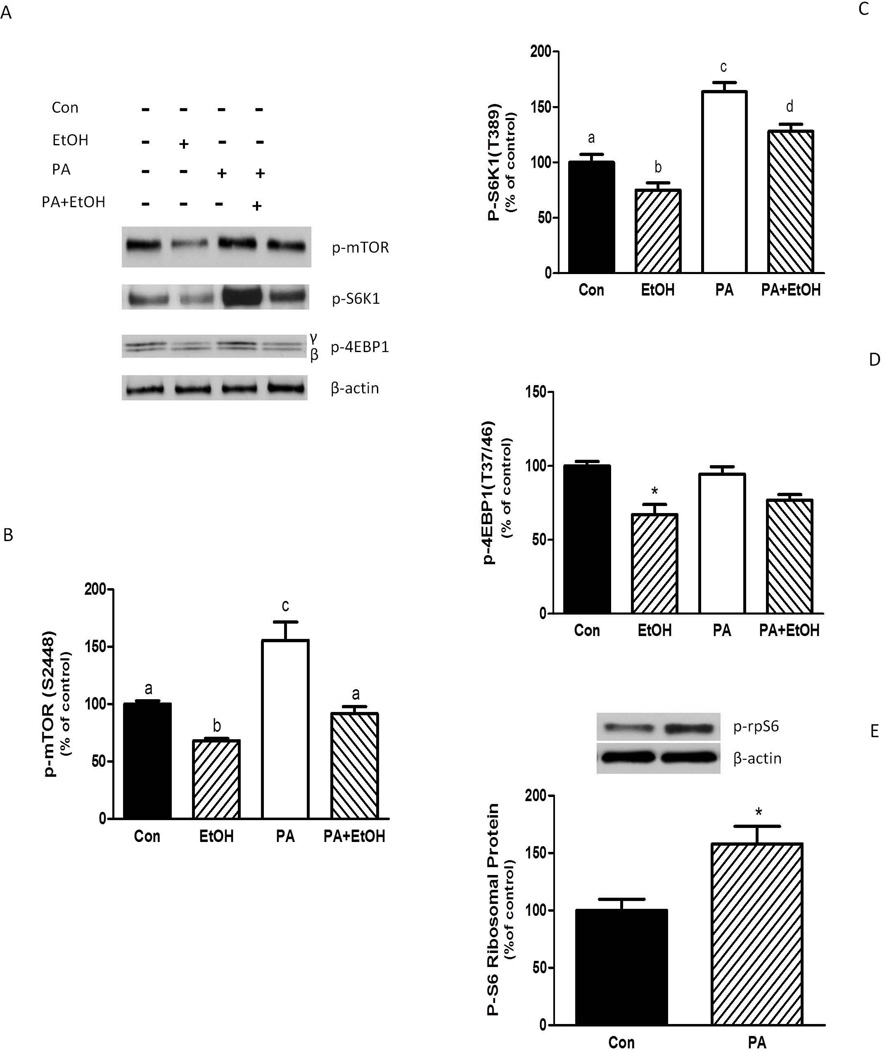
PA was also found to affect the activity of mTORC1. Exposure to this compound increased the phosphorylation of mTOR relative to control levels (Fig. 6A and 6B) and it blocked the EtOH-induced decrease in mTOR phosphorylation. In addition, PA increased S6K1 phosphorylation and it also countered the adverse effects of EtOH (Fig. 6A and 6C). As such, S6K1 phosphorylation remained above control level in the presence of both agents. In contrast, PA did not affect the phosphorylation of the mTORC1 target protein 4E-BP1 (Fig. 6A and 6D), suggesting specificity in the targeting of PA-induced mTORC1 activation. To verify the effect of PA on S6K1 activity, we assessed the phosphorylation state of ribosomal protein (rp) S6, a physiologically relevant S6K1 substrate. Treatment of myocytes with PA increased rpS6 phosphorylation (Fig. 6E).
Fig. 6.
PA increases S6K1, rpS6 and mTOR phosphorylation. C2C12 myocytes were incubated in the presence or absence of EtOH as described in Fig.5. Equal amounts of protein from cell lysates were analyzed via Western blotting using antibodies against phosphorylated (p)-mTOR, S6K1, and 4E-BP1 (A).Quantified data were plotted on a bar graph (B–D). (E) Cell extracts were analyzed using antibody recognize p-ribosomal protein (rp) S6. The data shown are mean ± SE of 4 independent experiments consisting of 4 replicate samples per experiment. Groups with different letters are significantly different from one another (* P< 0.05). Groups with the same letters are not significantly different. *P< 0.05 versus the control values.
PA decreases AMPK/ TSC2 phosphorylation and increases mTOR binding with Rheb and Rag GTPases
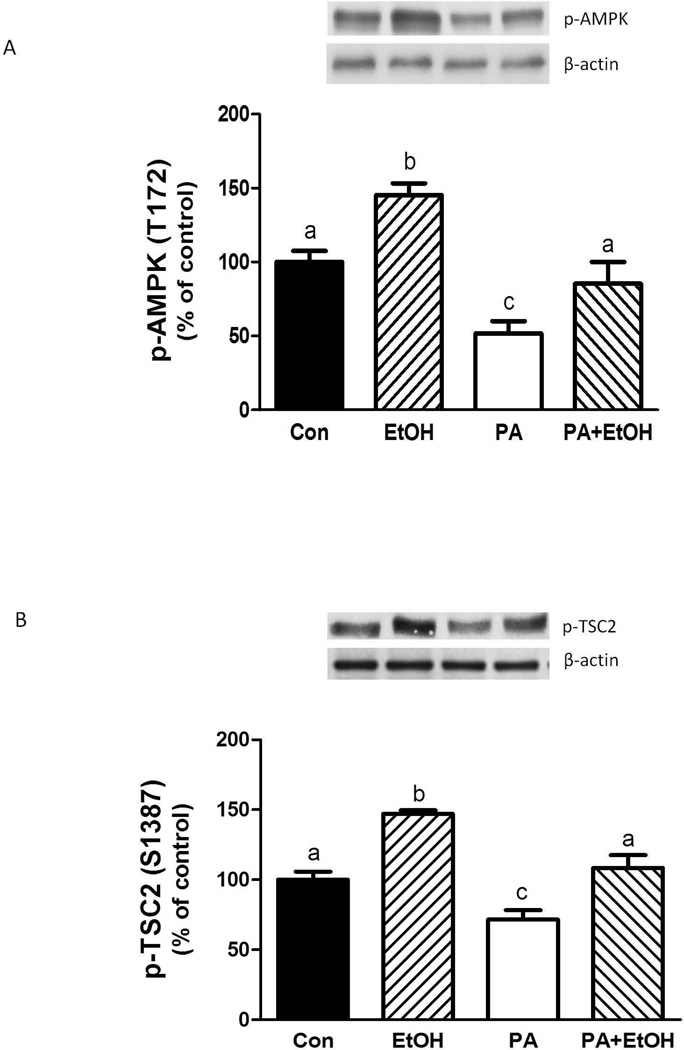
Previous studies have reported increased AMPK activity in response to various stressors. This increase, in turn, negatively regulates mTORC1 by enhancing the phosphorylation of TSC2, Raptor, and eukaryotic elongation factor (eEF)2 (Hong-Brown et al., 2007; 2010; Inoki et al., 2003b). As illustrated in Figure 7A, PA decreased the phosphorylation of AMPK compared to control levels. In contrast, EtOH increased AMPK. Because TSC2 is a downstream target of AMPK, we assessed whether PA affected TSC2 phosphorylation. PA decreased TSC2 phosphorylation at S1387 relative to control values (Fig. 7B). In contrast, EtOH enhanced TSC2 phosphorylation at this site.
Fig. 7.
PA decreases AMPK/TSC2 phosphorylation. C2C12 myocytes were incubated overnight in the presence or absence of EtOH as described in Fig 5. Equal amounts of protein from cell lysates were analyzed via Western blotting using antibodies against phosphorylated (p) forms of AMPKα (A) and TSC2 (B). The data shown are mean ± SE of 3 independent experiments consisting of 3–4 replicate samples per experiment. Groups with different letters are significantly different from one another (P< 0.05). Groups with the same letters are not significantly different.
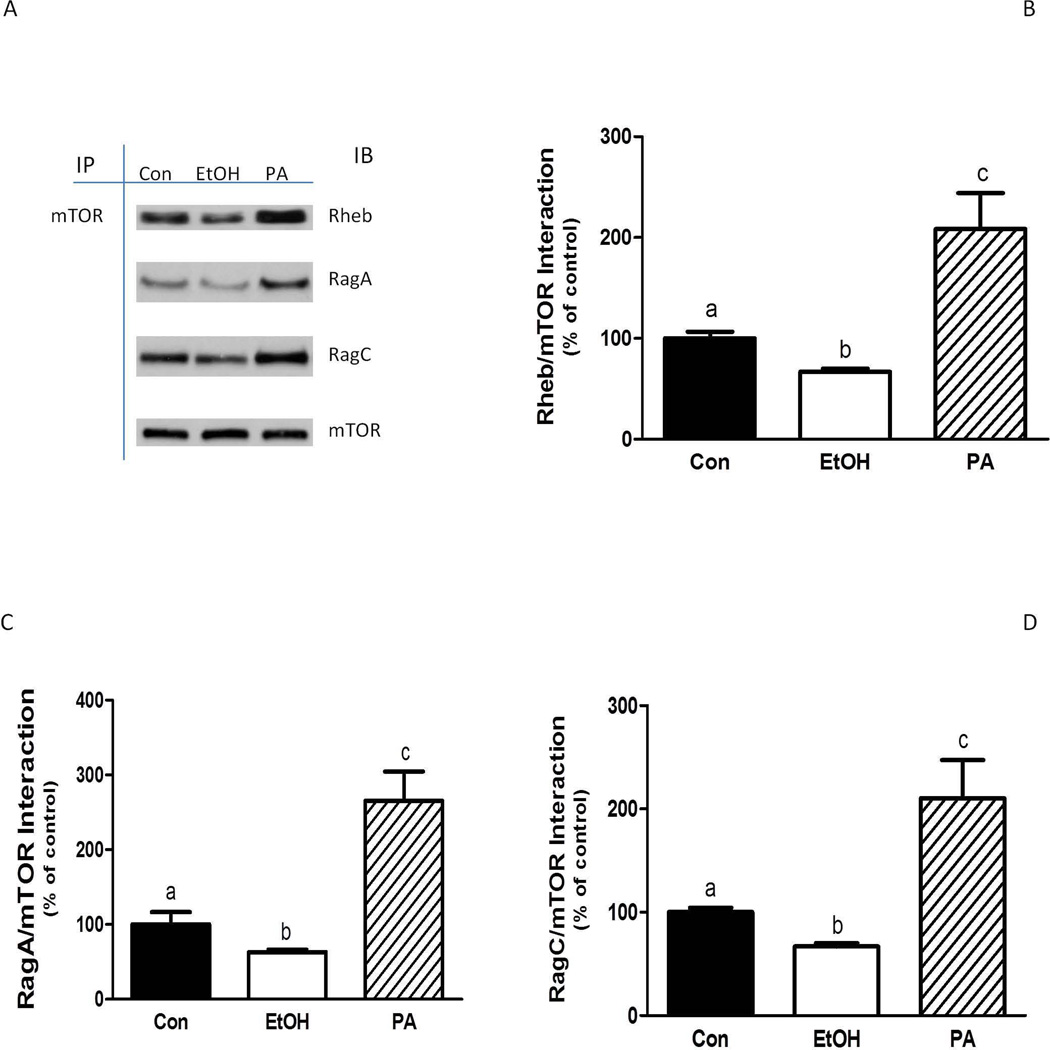
Alterations in TSC2 phosphorylation have been linked with changes in Rheb activity (Bai and Jiang, 2010) Therefore, we examined the effects of PA on the function of Rheb. Because protein-protein interactions are important in mediating signaling events, the association of Rheb with mTOR was assessed. The addition of PA increased the binding of Rheb with mTOR, while EtOH exposure decreased this interaction (Fig. 8A and 8B).
Fig. 8.
PA enhances the interaction of mTOR with Rheb and Rag GTPases. C2C12 myocytes were incubated in the presence or absence of EtOH as described in Fig. 5. mTOR was immunoprecipitated from equal amounts of cell extract and then immunoblotted with antibodies as indicated (A). Results were quantified as bar graphs (B–D) and normalized with immunoprecipitated (IP) mTOR that was assessed by immunoblotting. The data shown are mean ± SE of 3 independent experiments consisting of 3–4 replicate samples per experiment. Groups with different letters are significantly different from one another (* P< 0.05). Groups with the same letters are not significantly different.
The heterodimeric Rag GTPases have been implicated in EtOH and amino acid signaling to mTORC1 (Dickinson and Rasmussen, 2011; Hong-Brown et al., 2012a; Kim et al., 2008). Recently, it was demonstrated that the association of Rag and Raptor proteins results in the translocation of mTORC1 to the lysosome (Sancak et al., 2010). There, mTOR interacts with Rheb, resulting in the activation of the mTOR kinase. Incubation of cells with PA enhanced both RagA and RagC complex formation with mTOR, whereas EtOH decreased the interaction of these complexes (Fig. 8A, 8C and 8D). Similar results were also observed for the association between Raptor and the RagA-RagC complex (data not shown).
DISCUSSION
A number of studies have reported that PLD activity is elevated in response to mitogens such as growth factors, nutrients and hormones (Fang et al., 2001; Ma et al., 2010; Xu et al., 2011). Likewise, multiple stressors including serum withdrawal and glucose deprivation increase PLD activity (Kim et al., 2010; Lim et al., 2003; Zheng et al., 2006). These results are consistent with our data showing that EtOH increased PLD activity as well as the levels of the PLD metabolite PA. On the other hand, our results are in contrast with other findings where primary alcohols including EtOH and 1-butanol had inhibitory effect on these parameters (Fujita et al., 2008; Kotter and Klein, 1999). One possible explanation for this discrepancy is the difference in cell types examined. In addition, previous studies have utilized higher concentrations of EtOH, which may lead to toxic effects (Fujita et al., 2008; Tou and Gill, 2005).
In this study, we show that PLD and PA have important roles in regulating the activity of both mTORC1 and mTORC2. The mTORC2-mediated phosphorylation of Akt was dependent on PLD, with suppression of PLD decreasing in Akt (S473) phosphorylation. PLD inhibition also decreased mTORC1-dependent S6K1 (T389) phosphorylation, with these results being in agreement with previously published data (Toschi et al., 2009). On the other hand, our data suggest that PA is an important negative regulator of Akt (S473) and positive regulator of S6K1. These results also indicate a potential role for PLD and PA as part of the Akt/TSC2/Rheb signaling cascade which is involved in a number of physiological process (growth, survival, cytoskeletal assembly and vesicle trafficking) as well as disease states (cancer, diabetes and inflammation) (Foster, 2009; Guertin and Sabatini, 2005; Shaw and Cantley, 2006).
PLD and PA are regulated by a number of different factors, including phosphoinositides, small GTPases, PKC and serine-threonine kinases (Frohman et al., 1999; Jang et al., 2012). Here, we provide evidence that EtOH exerts its effect on PLD and PA via many of these same pathways. In particular, AMPK signaling was found to play a major role in regulating PLD, consistent with a study demonstrating that glucose deprivation increases PLD in an AMPK-dependent manner (Kim et al., 2010). Furthermore, the use of the AMPK inhibitor compound C abolished the EtOH-induced increase in PLD activity and it also prevented elevated TSC2 phosphorylation at S1387 (data not shown). Because TSC2 functions as a GAP for Rheb, inhibition of its activity promotes the binding of Rheb with mTOR, thereby enhancing mTORC1 function. Together, these data suggest that PLD is a downstream target of AMPK and TSC2. Rheb, on the other hand, has been proposed as an upstream regulator of PLD (Sun et al., 2008). Thus, it is possible that the effect of EtOH on mTOR is mediated via AMPK-TSC2/PLD signals.
We also observed that wortmannin blocked the ability of EtOH to stimulate PLD activity and AMPK phosphorylation indicating that AMPK is also controlled by the PI3K pathway. Because wortmannin can inhibit the action of both class I and class III members of PI3K, it is not clear which PI3K pathway is involved. However, previous data from our lab indicate that EtOH- induced decreases in mTORC1 activity was not mediated via class I PI3K (Hong-Brown et al., 2010). This conclusion was based on the observations whereby known downstream Akt targets, PRAS40 and TSC2 (T1462), were not involved in regulating mTORC1 activity. For example, EtOH-induced increases in Akt (T308 and S473) did not affect the extent of TSC2 phosphorylation at T1462. In addition, EtOH increased PRAS40 phosphorylation, but this did not enhance the interaction between PRAS40 and the negative regulatory protein 14-3-3. Hence, these data suggest that changes in AMPK/PLD function may be due to class III PI3K. In this regard, recent studies showed that PLD can be regulated by class III PI3K hVps34 (Xu et al., 2011).
Previous reports have noted that elevated PLD activity and PA levels have stimulatory effects on TORC1 (Hornberger et al., 2006; Winter et al., 2010). This is in agreement with our data in which myocytes incubated with PA increases mTOR, S6K1 and the ribosomal protein S6 phosphorylation. However, in the present study, EtOH increased PLD activity and PA levels, while concurrently decreasing mTORC1 function. Hence, decreased mTORC1 activity was not due to impairment of PLD. Increases in PA concentration were unexpected, owing to previous observations whereby PLD preferentially utilizes EtOH as a substrate rather than water, thereby forming the biologically inactive phosphatidylethanol (PE) instead of PA (Foster, 2010; Gustavsson, 1995). On the other hand, various stressors such as glucose deprivation or serum withdrawal have been shown to increase PLD activity (Kim et al., 2010; Zheng et al., 2006), consistent with our observations. Thus, elevations in PLD activity may serve to partially counterbalance the adverse effects of stress. Along these lines, it is possible that PLD and PA were not elevated to levels sufficient to overcome the negative effects of EtOH on mTORC1 activity. Indeed, in our PA experiments, the dose used was several-fold higher than concentrations observed in cells incubated with EtOH.
The mode of action whereby PLD and PA control mTOR activity has not been fully elucidated. However, changes in mTOR complex formation have been suggested to play an important role in regulating the effects of PLD. Recent studies reported that knockdown of PLD decreases the binding of mTOR to Rictor or Raptor (Toschi et al., 2009). Consistent with these results, we observed that PLD inhibition blocked EtOH-stimulated Akt phosphorylation, and by inference, prevented enhanced mTORC2 activity. This decreased mTORC2 activity may be due, at least in part, to changes in binding between Rictor, mTOR and mSin1. In addition, there was an increased interaction between Rictor and the negative regulatory proteins Deptor and 14-3-3. In the case of mTORC1, suppression of PLD produced similar effects as EtOH on Raptor interaction with its components. In contrast, PA appeared to regulate mTORC1 via changes in the interactions of mTOR with Rheb and Rag GTPase. The observed decrease in Akt phosphorylation by exogenous PA appears to be regulated by the mTORC1/2 negative feedback loop. Increased S6K1 phosphorylation resulted in higher levels of phosphorylated Rictor. However, in contract to published data (Dibble et al., 2009; Treins et al., 2010), there was a decreased binding of Rictor with mSin1 and 14-3-3. Therefore, the PA-induced decrease in Akt phosphorylation could not be due to the dampening effects of 14-3-3 binding (Bridges and Moorhead, 2005; Chen et al., 2011).
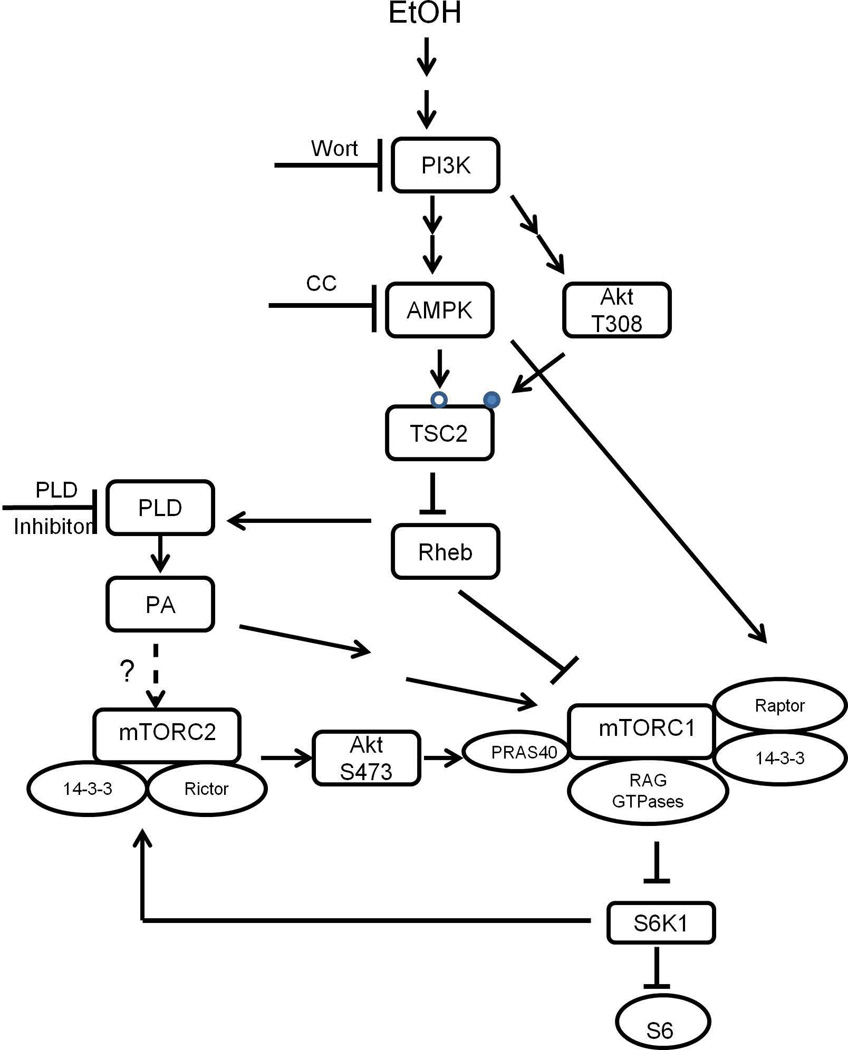
Based on the current result, we propose a model (Fig. 9) describing mechanisms whereby EtOH or PA affects mTOR signaling. EtOH activates AMPK, thereby leading to the phosphorylation and activation of TSC2 (S1387). TSC2 then inactivates Rheb, which impairs the interaction of mTOR with Rheb and Rag proteins. This leads to decreased mTORC1 activity towards S6K1. EtOH also activates a second mechanism involving increased Raptor phosphorylation. This results in increased association of this protein with the inhibitory protein 14-3-3. Diminishing S6K1 activity leads to activation of mTORC2 towards Akt (S473) via a negative feedback loop. PA appears to affect the same pathway, although this generally occurs in the opposite manner of EtOH. In summary, both EtOH and PA activate the AMPK/TSC2//PLD signaling cascade that regulates mTORC1 and mTORC2.
Fig. 9.
Proposed Model for EtOH and PA regulation of mTORC1 and mTORC2 signaling. EtOH stimulates PLD activity and PA content and this process is mediated via multiple signaling cascades. EtOH increases AMPK phosphorylation and activity. This results in upregulation of TSC2 phosphorylation at the S1387 site (open circle), but it has no effect on the T1462 residue (closed circle). Activation of TSC2 at S1387 leads to a decreased Rheb /mTOR interaction and decreased mTORC1 activity towards S6K1. Decreased S6K1 phosphorylation enhanced mTORC2 activity and increased Akt (S473) phosphorylation. PLD and PA regulate the same pathway, albeit in an opposite manner. EtOH and PA affect both mTOR complexes via a PI3K/AMPK/TSC2/PLD signaling cascade.
In conclusion, our results indicate that EtOH increases PLD activity and PA levels. Hence, the inhibitory effect of EtOH on mTORC1 cannot be attributed to an inhibition of PLD function. Suppression of PLD prevented EtOH-induced Akt (S473) phosphorylation, and this was correlated with enhanced binding of Rictor to various mTORC2 components and 14-3-3. EtOH and PA both regulated mTORC1 via PI3K/AMPK/TSC2/PLD signaling cascades, albeit in the opposite manner. The addition of PA to myocytes increased mTORC1-dependent S6K1 phosphorylation, and this was associated with increased binding of mTOR with Rheb and Rag GTPase. In contrast, PA decreased mTORC2-dependent phosphorylation of Akt on S473, although the mechanisms mediating this effect are unknown. Taken together, these data extend our knowledge regarding the connection between EtOH and PLD activity. While additional studies are required to fully elucidate this process, stimulation of PLD and PA by EtOH may partially counterbalance the adverse consequences of this drug. In addition, the contrasting effects of PA on mTORC1 and mTORC2 may indicate a role for this compound in activating an mTORC1/2 feedback loop.
ACKNOWLEDGMENTS
This study was supported by National Institute of Health Grant R37 AA-011290.
The abbreviations used are
- EtOH
ethanol
- PLD
phospholipase D
- PA
phosphatidic acid
- mTORC
mammalian target of rapamycin complex
- mLST8/GβL
mammalian lethal with sec13 protein 8/ G protein β-subunit-like protein
- Raptor
regulatory associated protein of mTOR
- PRAS40
proline-rich Akt substrate 40 kDa
- Deptor
DEP- domain-containing mTOR-interacting protein
- Rictor
rapamycin-insensitive companion of mTOR
- mSin1
mammalian stress-activated protein kinase (SAPK)-interacting protein
- Protor/PRR5
protein observed with rictor/proline-rich repeat protein 5
- PI3K
Phosphoinositide 3-kinase
- AMPK
AMP-activated protein kinase
- TSC
tuberous sclerosis
- GAP
GTPase activator protein
- Rheb
Ras homolog enriched in brain
- Rag
Ras-related GTPase
- S6K
Ribosomal S6 kinase
- rpS6
ribosomal protein S6
- 4EBP
eukaryotic initiation factor 4E binding protein
REFERENCES
- Bai X, Jiang Y. Key factors in mTOR regulation. Cell Mol Life Sci. 2010;67:239–253. doi: 10.1007/s00018-009-0163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibollet-Bahena O, Almazan G. IGF-1-stimulated protein synthesis in oligodendrocyte progenitors requires PI3K/mTOR/Akt and MEK/ERK pathways. J Neurochem. 2009;109:1440–1451. doi: 10.1111/j.1471-4159.2009.06071.x. [DOI] [PubMed] [Google Scholar]
- Bridges D, Moorhead GB. 14-3-3 proteins: a number of functions for a numbered protein. Sci STKE. 2005;2005:re10. doi: 10.1126/stke.2962005re10. [DOI] [PubMed] [Google Scholar]
- Chen S, Synowsky S, Tinti M, MacKintosh C. The capture of phosphoproteins by 14-3-3 proteins mediates actions of insulin. Trends Endocrinol Metab. 2011;22:429–436. doi: 10.1016/j.tem.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Dibble CC, Asara JM, Manning BD. Characterization of Rictor phosphorylation sites reveals direct regulation of mTOR complex 2 by S6K1. Mol Cell Biol. 2009;29:5657–5670. doi: 10.1128/MCB.00735-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson JM, Rasmussen BB. Essential amino acid sensing, signaling, and transport in the regulation of human muscle protein metabolism. Curr Opin Clin Nutr Metab Care. 2011;14:83–88. doi: 10.1097/MCO.0b013e3283406f3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop EA, Tee AR. Mammalian target of rapamycin complex 1: signalling inputs, substrates and feedback mechanisms. Cell Signal. 2009;21:827–835. doi: 10.1016/j.cellsig.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Faghiri Z, Bazan NG. PI3K/Akt and mTOR/p70S6K pathways mediate neuroprotectin D1-induced retinal pigment epithelial cell survival during oxidative stress-induced apoptosis. Exp Eye Res. 2010;90:718–725. doi: 10.1016/j.exer.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2001;294:1942–1945. doi: 10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
- Foster DA. Phosphatidic acid signaling to mTOR: signals for the survival of human cancer cells. Biochim Biophys Acta. 2009;1791:949–955. doi: 10.1016/j.bbalip.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DA. Reduced mortality and moderate alcohol consumption: the phospholipase D-mTOR connection. Cell Cycle. 2010;9:1291–1294. doi: 10.4161/cc.9.7.11145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KG, Fingar DC. Mammalian target of rapamycin (mTOR): conducting the cellular signaling symphony. J Biol Chem. 2010;285:14071–14077. doi: 10.1074/jbc.R109.094003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman MA, Sung TC, Morris AJ. Mammalian phospholipase D structure and regulation. Biochim Biophys Acta. 1999;1439:175–186. doi: 10.1016/s1388-1981(99)00093-1. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Hiroyama M, Sanbe A, Yamauchi J, Murase S, Tanoue A. ETOH inhibits embryonic neural stem/precursor cell proliferation via PLD signaling. Biochem Biophys Res Commun. 2008;370:169–173. doi: 10.1016/j.bbrc.2008.03.060. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Sabatini DM. An expanding role for mTOR in cancer. Trends Mol Med. 2005;11:353–361. doi: 10.1016/j.molmed.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Gustavsson L. ESBRA 1994 Award Lecture. Phosphatidylethanol formation: specific effects of ethanol mediated via phospholipase D. Alcohol Alcohol. 1995;30:391–406. [PubMed] [Google Scholar]
- Hong-Brown LQ, Brown CR, Huber DS, Lang CH. Alcohol and indinavir adversely affect protein synthesis and phosphorylation of MAPK and mTOR signaling pathways in C2C12 myocytes. Alcohol Clin Exp Res. 2006;30:1297–1307. doi: 10.1111/j.1530-0277.2006.00157.x. [DOI] [PubMed] [Google Scholar]
- Hong-Brown LQ, Brown CR, Huber DS, Lang CH. Alcohol regulates eukaryotic elongation factor 2 phosphorylation via an AMP-activated protein kinase-dependent mechanism in C2C12 skeletal myocytes. J Biol Chem. 2007;282:3702–3712. doi: 10.1074/jbc.M606593200. [DOI] [PubMed] [Google Scholar]
- Hong-Brown LQ, Brown CR, Kazi AA, Huber DS, Pruznak AM, Lang CH. Alcohol and PRAS40 knockdown decrease mTOR activity and protein synthesis via AMPK signaling and changes in mTORC1 interaction. J Cell Biochem. 2010;109:1172–1184. doi: 10.1002/jcb.22496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong-Brown LQ, Brown CR, Kazi AA, Navaratnarajah M, Lang CH. Rag GTPases and AMPK/TSC2/Rheb mediate the differential regulation of mTORC1 signaling in response to alcohol and leucine. Am J Physiol Cell Physiol. 2012a;302:C1557–C1565. doi: 10.1152/ajpcell.00407.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong-Brown LQ, Brown CR, Navaratnarajah M, Huber DS, Lang CH. Alcohol-induced modulation of rictor and mTORC2 activity in C2C12 myoblasts. Alcohol Clin Exp Res. 2011;35:1445–1453. doi: 10.1111/j.1530-0277.2011.01480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong-Brown LQ, Kazi AA, Lang CH. Mechanisms mediating the effects of alcohol and HIV anti-retroviral agents on mTORC1, mTORC2 and protein synthesis in myocytes. World J Biol Chem. 2012b;3:110–120. doi: 10.4331/wjbc.v3.i6.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberger TA, Chu WK, Mak YW, Hsiung JW, Huang SA, Chien S. The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signaling in skeletal muscle. Proc Natl Acad Sci U S A. 2006;103:4741–4746. doi: 10.1073/pnas.0600678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003a;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4:648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003b;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Jang JH, Lee CS, Hwang D, Ryu SH. Understanding of the roles of phospholipase D and phosphatidic acid through their binding partners. Prog Lipid Res. 2012;51:71–81. doi: 10.1016/j.plipres.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol. 2008;10:935–945. doi: 10.1038/ncb1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Park JM, Yea K, Kim HW, Suh PG, Ryu SH. Phospholipase D1 mediates AMP-activated protein kinase signaling for glucose uptake. PLoS One. 2010;5:e9600. doi: 10.1371/journal.pone.0009600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotter K, Klein J. Ethanol inhibits astroglial cell proliferation by disruption of phospholipase D-mediated signaling. J Neurochem. 1999;73:2517–2523. doi: 10.1046/j.1471-4159.1999.0732517.x. [DOI] [PubMed] [Google Scholar]
- Lehman N, Ledford B, Di Fulvio M, Frondorf K, McPhail LC, Gomez-Cambronero J. Phospholipase D2-derived phosphatidic acid binds to and activates ribosomal p70 S6 kinase independently of mTOR. Faseb J. 2007;21:1075–1087. doi: 10.1096/fj.06-6652com. [DOI] [PubMed] [Google Scholar]
- Lim HK, Choi YA, Park W, Lee T, Ryu SH, Kim SY, Kim JR, Kim JH, Baek SH. Phosphatidic acid regulates systemic inflammatory responses by modulating the Akt-mammalian target of rapamycin-p70 S6 kinase 1 pathway. J Biol Chem. 2003;278:45117–45127. doi: 10.1074/jbc.M303789200. [DOI] [PubMed] [Google Scholar]
- Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10:457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- Ma WN, Park SY, Han JS. Role of phospholipase D1 in glucose-induced insulin secretion in pancreatic Beta cells. Exp Mol Med. 2010;42:456–464. doi: 10.3858/emm.2010.42.6.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memmott RM, Dennis PA. Akt-dependent and -independent mechanisms of mTOR regulation in cancer. Cell Signal. 2009;21:656–664. doi: 10.1016/j.cellsig.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier R, Lantier L, Leclerc J, Sotiropoulos A, Foretz M, Viollet B. Antagonistic control of muscle cell size by AMPK and mTORC1. Cell Cycle. 2011;10:2640–2646. doi: 10.4161/cc.10.16.17102. [DOI] [PubMed] [Google Scholar]
- O'Neil TK, Duffy LR, Frey JW, Hornberger TA. The role of phosphoinositide 3-kinase and phosphatidic acid in the regulation of mammalian target of rapamycin following eccentric contractions. J Physiol. 2009;587:3691–3701. doi: 10.1113/jphysiol.2009.173609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Djerdjouri B, Raoul-Des-Essarts Y, Dang PM, El-Benna J, Perianin A. Protein kinase B (AKT) mediates phospholipase D activation via ERK1/2 and promotes respiratory burst parameters in formylpeptide-stimulated neutrophil-like HL-60 cells. J Biol Chem. 2010;285:32055–32063. doi: 10.1074/jbc.M110.171058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- Sun Y, Fang Y, Yoon MS, Zhang C, Roccio M, Zwartkruis FJ, Armstrong M, Brown HA, Chen J. Phospholipase D1 is an effector of Rheb in the mTOR pathway. Proc Natl Acad Sci U S A. 2008;105:8286–8291. doi: 10.1073/pnas.0712268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toschi A, Lee E, Xu L, Garcia A, Gadir N, Foster DA. Regulation of mTORC1 and mTORC2 complex assembly by phosphatidic acid: competition with rapamycin. Mol Cell Biol. 2009;29:1411–1420. doi: 10.1128/MCB.00782-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tou JS, Gill JS. Lysophosphatidic acid increases phosphatidic acid formation, phospholipase D activity and degranulation by human neutrophils. Cell Signal. 2005;17:77–82. doi: 10.1016/j.cellsig.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Treins C, Warne PH, Magnuson MA, Pende M, Downward J. Rictor is a novel target of p70 S6 kinase-1. Oncogene. 2010;29:1003–1016. doi: 10.1038/onc.2009.401. [DOI] [PubMed] [Google Scholar]
- Winter JN, Fox TE, Kester M, Jefferson LS, Kimball SR. Phosphatidic acid mediates activation of mTORC1 through the ERK signaling pathway. Am J Physiol Cell Physiol. 2010;299:C335–C344. doi: 10.1152/ajpcell.00039.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Xu L, Salloum D, Medlin PS, Saqcena M, Yellen P, Perrella B, Foster DA. Phospholipase D mediates nutrient input to mammalian target of rapamycin complex 1 (mTORC1) J Biol Chem. 2011;286:25477–25486. doi: 10.1074/jbc.M111.249631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Rodrik V, Toschi A, Shi M, Hui L, Shen Y, Foster DA. Phospholipase D couples survival and migration signals in stress response of human cancer cells. J Biol Chem. 2006;281:15862–15868. doi: 10.1074/jbc.M600660200. [DOI] [PubMed] [Google Scholar]