Figure 8.
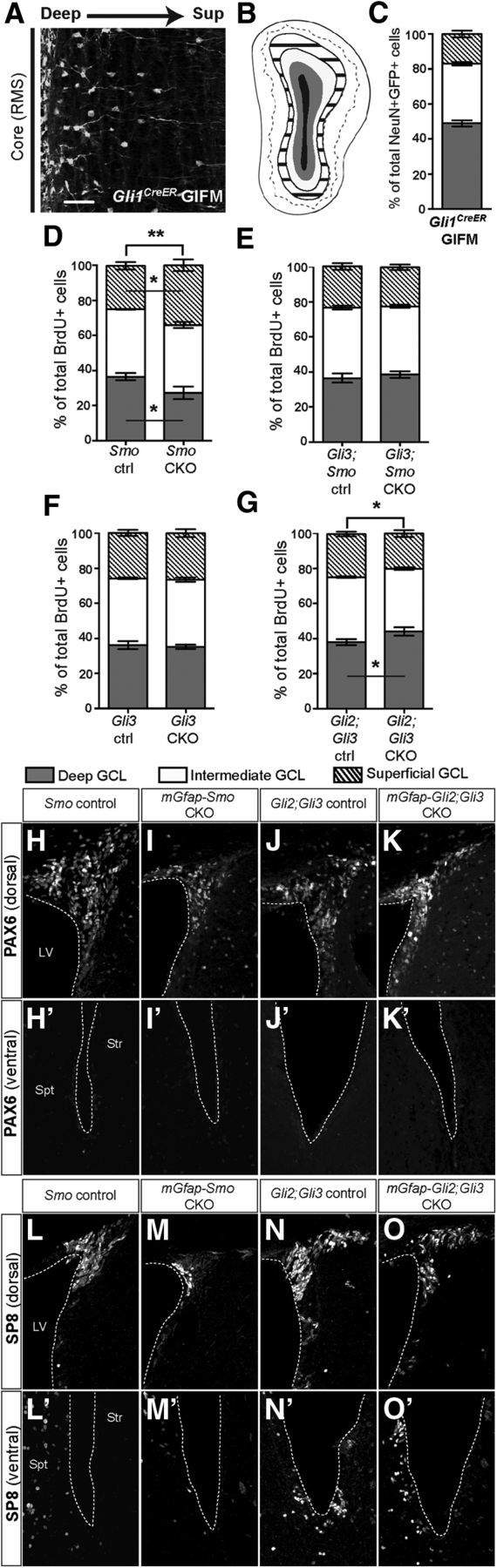
SHH is required postnatally to regulate the normal proportions of interneurons that settle in the deep to superficial axis of the OB granule layer. A, Coronal section through the OB of a Gli1CreER/+; R26yfp/yfp mouse 30 d after tamoxifen treatment (Gli1CreER GIFM) at 3 months shows that fate-mapped interneuron progeny localize preferentially to the region closest to the RMS (core). Scale bar, 50 μm. B, Schematic of an OB granule cell layer subdivided into deep (gray area), intermediate (white area), and superficial (striped area) domains based on lines drawn to demarcate the borders between domains 1/3 and 2/3 of the radius between the outline of the RMS (core; in black) and the outer rim of the GCL. C, Quantitative representation of the distribution of interneurons derived from Gli1-expressing NSCs shows that the majority of Gli1CreER fate-mapped mature (NeuN+) interneurons localize to the deep and intermediate GCL domains. D–G, Quantitation of the distribution of newly generated (BrdU+) interneurons within the GCL shows that in mGfap-Smo CKOs (D) there is a shift toward generating more superficial layer interneurons and a reduction in deep OB interneurons (2-way ANOVA with Sidak's post hoc test; ANOVA interaction p value **p = 0.0053; mean ± SEM), whereas in mGfap-Gli3;Smo CKOs (E) the distribution is not altered compared with controls (2-way ANOVA with Sidak's post hoc test; mean ± SEM). Loss of Gli3 alone (F) is not sufficient to induce a change in the OB interneuron distribution compared with controls (2-way ANOVA with Sidak's post hoc test; mean ± SEM). In contrast, conditional ablation of both Gli2 and Gli3 in mGfap-Gli2;Gli3 CKOs (G) results in a slight increase in the frequency of generation of deep-layer OB interneurons compared with controls (ANOVA interaction p value *p = 0.02; mean ± SEM). H–K', Staining for the dorsal molecular marker PAX6 in mGfap-Smo CKOs (H, H', I, I') and mGfap-Gli2;Gli3 CKOs (J, J', K, K') revealed no change in PAX6 expression and hence in progenitor respecification along the dorsal–ventral axis of the SVZ. Analysis for the putative repatterning of the SVZ in the same two mutants using the dorsally expressed marker SP8 yielded similar results (L–O'). A minimum of n = 3 littermate controls and 3 mutants were used for the analysis of each experimental group.
