FIG 1 .
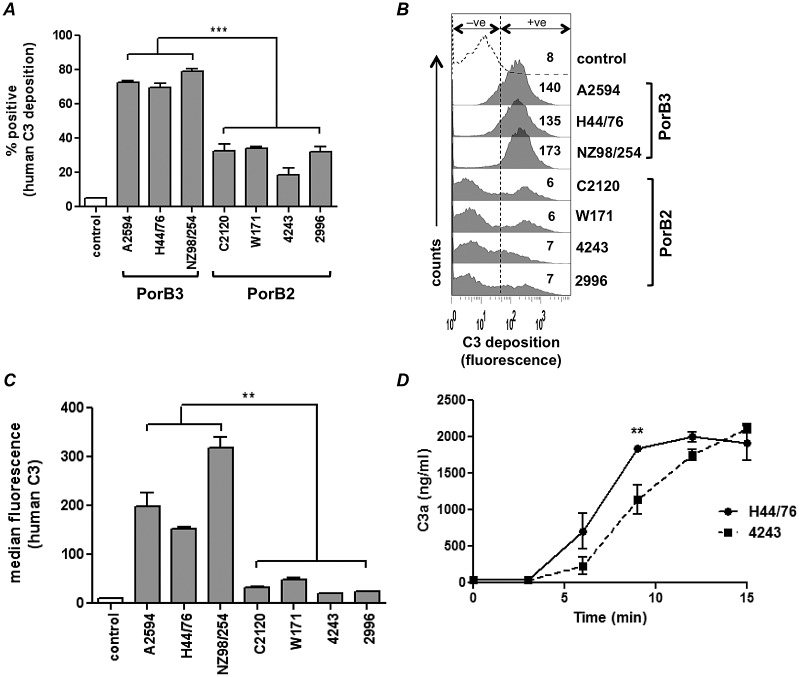
Regulation of the human AP by PorB2-expressing N. meningitidis. Seven strains of N. meningitidis that lacked capsular polysaccharide, LOS sialic acid, and fHbp and NspA expression (quadruple mutants) were screened for AP-mediated C3 deposition with NHS-Mg/EGTA (20% [vol/vol]). (A) C3 deposition measured by FACS analysis. The percentage of positive events relative to control organisms incubated with heat-inactivated serum (gated to yield 5% of positive events in the negative-control sample as shown in Fig. 1B [+ve]) is represented on the y axis. Each bar represents the standard error of the mean (SEM) of the results of three independent experiments. ***, P < 0.001 (1-way ANOVA with Tukey’s posttest for pairwise comparisons). (B) Histograms from an experiment representative of those described for panel A; data represent the median fluorescence of C3 binding of the entire bacterial population. (C) AP regulation by PorB2-expressing strains revealed using purified AP components. Quadruple mutants were incubated with purified C3 (500 µg/ml), factor B (100 µg/ml), factor D (2 µg/ml), and factor H (100 µg/ml). C3b deposited on bacteria was measured by FACS analysis. Controls were bacteria incubated with C3 alone. The amount of deposited C3b is represented as median fluorescence (y axis). Each bar represents the mean (range) of the results of two separate experiments. **, P < 0.01. (D) 4243 (PorB2) activates the AP at a lower rate than H44/76 (PorB3). Quadruple mutants of 4243 (PorB2) and H44/76 (PorB3) were incubated with NHS-Mg/EGTA. Supernatants collected at the times indicated (x axis) were assayed for C3a by enzyme-linked immunosorbent assay (ELISA). Each datum point represents the SEM of the results of three independent experiments. **, P < 0.01 (2-way ANOVA).