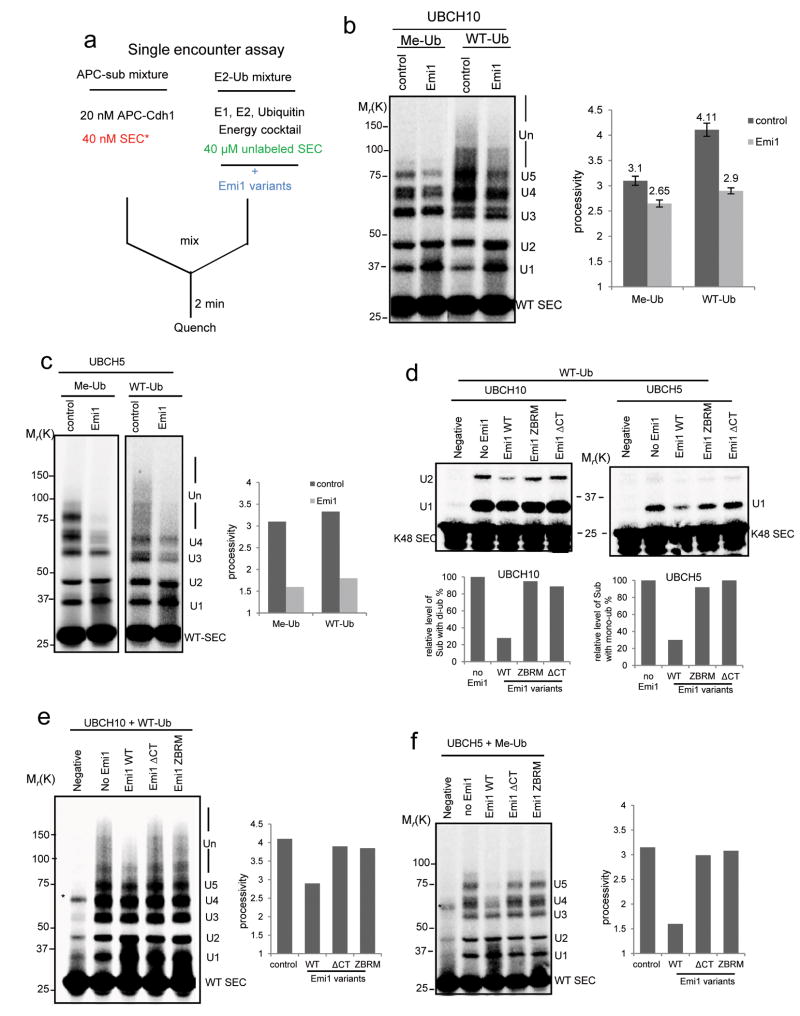
Figure 4.
Emi1 inhibits the slow process of ubiquitin transfer from charged UBCH10 or UBCH5.
(a) Assay was designed for testing how Emi1 affects ubiquitin transfer from charged E2. Emi1 variants were pre-incubated with “E2-Ub” mixture in the single encounter assay to study the possible function of Emi1 against E2 activity. (b) 2 μM Emi1 was pre-incubated with “E2-Ub” to test how Emi1 affects the ubiquitylation of 33P radiolabeled WT securin catalyzed by 1μM UBCH10. Methylated ubiquitin (Me-Ub) was used to measure the processivity of multiple-monoubiquitylation; Wild type ubiquitin (WT-Ub) was used to measure the processivity from both monoubiquitylation and chain extension. The processivity of reactions was calculated, and the mean value ± s.d. of five independent experiments is shown. Note that the processivity contributed by chain extension in control and Emi1 (+) group is 1.01 (4.11-3.1) and 0.25 (2.9-2.65) respectively. (c) 2 μM Emi1 was pre-incubated with “E2-methylated Ub” to test how Emi1 affects the ubiquitylation of 33P radiolabeled WT securin catalyzed by 1 μM UBCH5. (d) Emi1 variants were pre-incubated with “E2-WT Ub” to evaluate their effects on ubiquitylation of the single-lysine securin (K48 SEC) by either UBCH10 or UBCH5. The reaction products by UBCH10 with di-ubiquitin and products by UBCH5 with mono-ubiquitin were quantified respectively. (e) Emi1 variants were pre-incubated with “UBCH10-WT Ub” to examine their inhibitory activities on the polyubiquitylation of WT securin. (f) Emi1 variants were pre-incubated with “UBCH5-methylated Ub” to study their inhibitory activities on multiple monoubiquitylation of WT securin. All the reactions above were analyzed by autoradiography. * indicates nonspecific band.