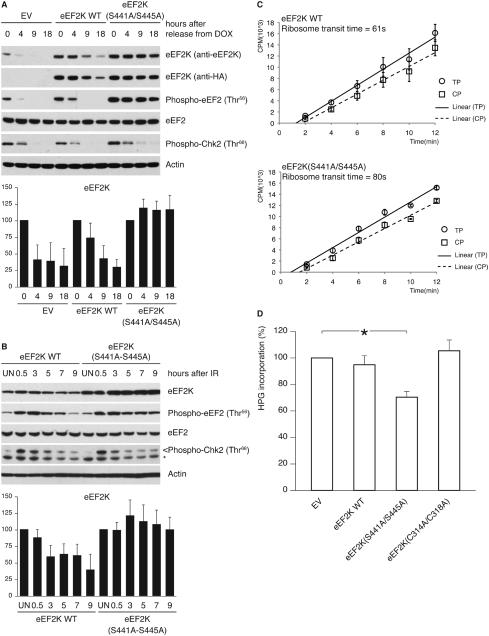
Figure 6. Inability to degrade eEF2K delays the resumption of translation elongation during silencing of the DNA damage checkpoint.
(A) U2OS cells, transduced with retroviruses expressing HA-tagged wild type eEF2K or eEF2K(S441A/S445A), were synchronized in G2, doxorubicin-pulsed and collected at the indicated times. Cell extracts were analyzed by immunoblotting. (B) U2OS cells, transduced as in (A), were exposed to ionizing radiation. Cell extracts were analyzed by immunoblotting. The graphs illustrate the quantification by densitometry of eEF2K abundance relative to the amount at time 0 (A) or in untreated sample (B). (C) U2OS cells, transduced as in (A), were collected 12 hours after ionizing radiation. Incorporation of [35S]methionine into total proteins (TP; circles) or into completed peptides released from ribosomes (CP; squares) is shown. Comparison of ribosome transit time for eEF2K WT and eEF2K(S441A/S445A) expressing cells by Student’s t-test gives P<0.005.
(D) Transduced U2OS cells were treated as in (A). Sixteen hours after the doxorubicin pulse, cells were labeled with L-homopropargylglycine. Incorporation of L-homopropargylglycine was detected using Click-iT technology. Results are the mean ± SD (n=3; *P<0.01 comparing to EV by one-way ANOVA and Dunnett’s post-hoc test). Data represent at least three independent experiments.