Table 2.
Substrate scope.
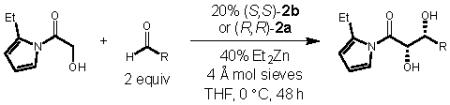
| entrya | R | yield (%)b |
dr (syn/anti)c |
ee (%)d | |
|---|---|---|---|---|---|
| 1 | Cyclohexyl | 3c | 77 | 11:1 | 92 |
| 2 | i-Pr | 3d | 79 | 7.1:1 | 90 |
| 3 | CH(CH2CH3)2 | 3e | 67 | 5.2:1 | 88 |
| 4 | CH2CH(CH3)2 | 3f | 86 | 3.7:1 | 87 |
| 5 | n-Butyl | 3g | 56 | 3.9:1 | 87 |
| 6 | CH2CH2Ph | 3h | 72 | 2.6:1 | 84 |
| 7 |
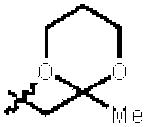
|
3i | 84 | 3.6:1 | 88 |
| 8e | t-Butyl | 3j | 51 | 15:1 | −75 |
| 9e |
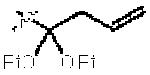
|
3k | 66 | 19:1 | −74 |
| 10e |
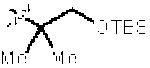
|
3l | 57 | 16:1 | −72 |
| 11e,f | C6H5 | 3m | 65 | 1.7:1 | −92 |
| 12f | p-ClC6H4 | 3n | 59 | 1.8:1 | 91 |
| 13f |
|
3o | 59 | 1.6:1 | 87 |
Reactions performed using 0.2 mmol 1b, 2.0 equiv aldehyde, 0.025 g 4 Å molecular sieves, 20% (S, S)-2b or (R,R)-2a, and 40% Et2Zn in THF (0.4 M) at 0 °C for 48 h.
Isolated Yield.
Determined by 1H NMR of crude reaction mixture.
Determined by chiral HPLC analysis
20 mol% (R,R)-3a was used.
Run for 72 h at −15 °C
