Figure 7.
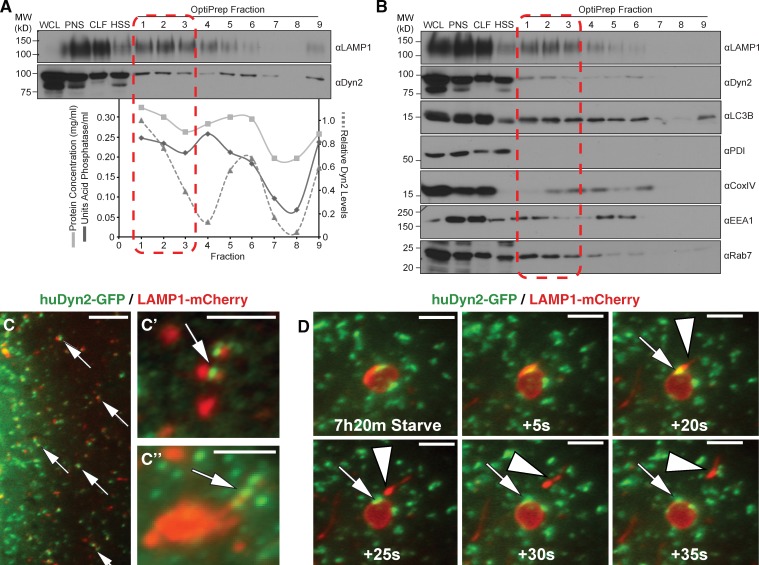
Dyn2 associates with autolysosomal tubules. (A and B) Subcellular density gradient fractionation of Hep3B hepatocytes starved for 2 h in HBSS and treated with 40 µM Dynasore, to induce tubule formation, as in Fig. 5. Cells were lysed (WCL), and the post-nuclear supernatant (PNS) was pelleted by centrifugation to produce a crude lysosomal fraction (CLF) and high-speed supernatant (HSS). The CLF was subsequently loaded onto an 8–27% discontinuous iodixanol (OptiPrep) gradient for separation by ultracentrifugation. Nine fractions were collected from the top of the gradient and blotted for Dyn2 and the lysosomal resident protein, LAMP1. Lysosomal acid phosphatase activity roughly correlates with LAMP1 protein levels in each fraction. Levels of Dyn2 are highest in fraction 2, corresponding with both the peak levels of LAMP1 and lysosomal activity. (A) The data shown are from a single representative experiment out of three repeats. (B) Blotting for subcellular components shows that Dyn2 is specific for these same fractions, unlike other organelle markers such as EEA1 (early endosomes) and COXIV (mitochondria). (C and D) Dyn2 localizes to the surface of LAMP1-positive compartments. Fluorescence imaging of Hep3B hepatocytes transfected with Dyn2-GFP and LAMP1-mCherry under resting (C and C′) or starvation (D) conditions. (C′′) Dyn2-GFP localizing to LAMP1-labeled lysosome structures under starvation conditions. Arrows indicate regions of protein colocalization. Dyn2 (arrows) is present at the site of scission of LAMP1-positive tubules (arrowhead) from large autolysosomal structures. Bars: (C) 5 µM; (C′, C′′, and D) 3 µM.