Figure 4.
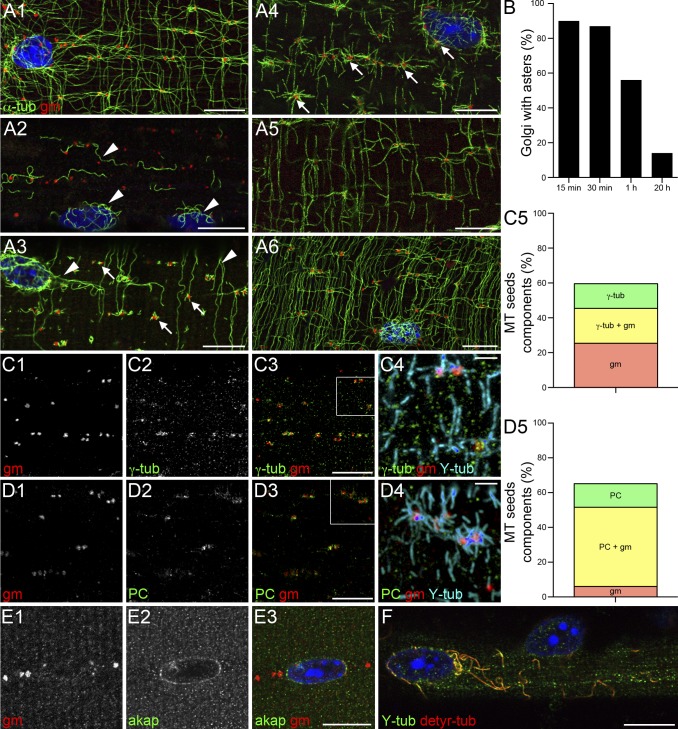
MTs are nucleated on Golgi elements that concentrate γ-tubulin and pericentrin. To investigate MT nucleation, plated FDB fibers were treated with NZ to depolymerize MTs and fixed after different periods of recovery. They were then stained with anti–α-tubulin (α-tub) and anti-GM130 (gm) to label MTs and Golgi elements. In a control fiber (A1), Golgi elements are along MTs, especially at crossings, and around nuclei. After NZ, before recovery (A2), only a few curly MTs remain (arrowheads). These contain both detyrosylated and tyrosylated tubulins (detyr-tub and Y-tub; F), indicating the presence of both stable and dynamic MTs. In the first minute of recovery, NZ-resistant MTs elongate (A3, arrowheads) and MT seeds appear around Golgi elements (A3, arrows). A few minutes later, full asters centered on Golgi elements become prominent (A4, arrows). MTs then progressively reform a network (A5 and A6). During early recovery, ∼90% of Golgi elements are at the center of MT asters; at steady-state, only ∼10% are (B; data are from a single representative experiment out of three; n = 100, from five fibers, for each time point). Fibers at early stages of recovery (as shown in A3) were stained with anti-GM130 and with antibodies against γ-tubulin (γ-tub), pericentrin, and AKAP450. γ-Tubulin and pericentrin are concentrated on Golgi elements of MT seeds (C1–C4 and D1–D4); AKAP450 is only detected around nuclei (E1–E3). Most MT seeds are associated with Golgi elements ± γ-tubulin and some with γ-tubulin alone (C5; Table 2). Similar results are obtained for quantitation with pericentrin (D5; Table 3; two independent experiments, nine fibers, 400 MT seeds for C5, and 322 MT seeds for D5). Bars: (A–F) 10 µm; (C4 and D4) 2 µm.