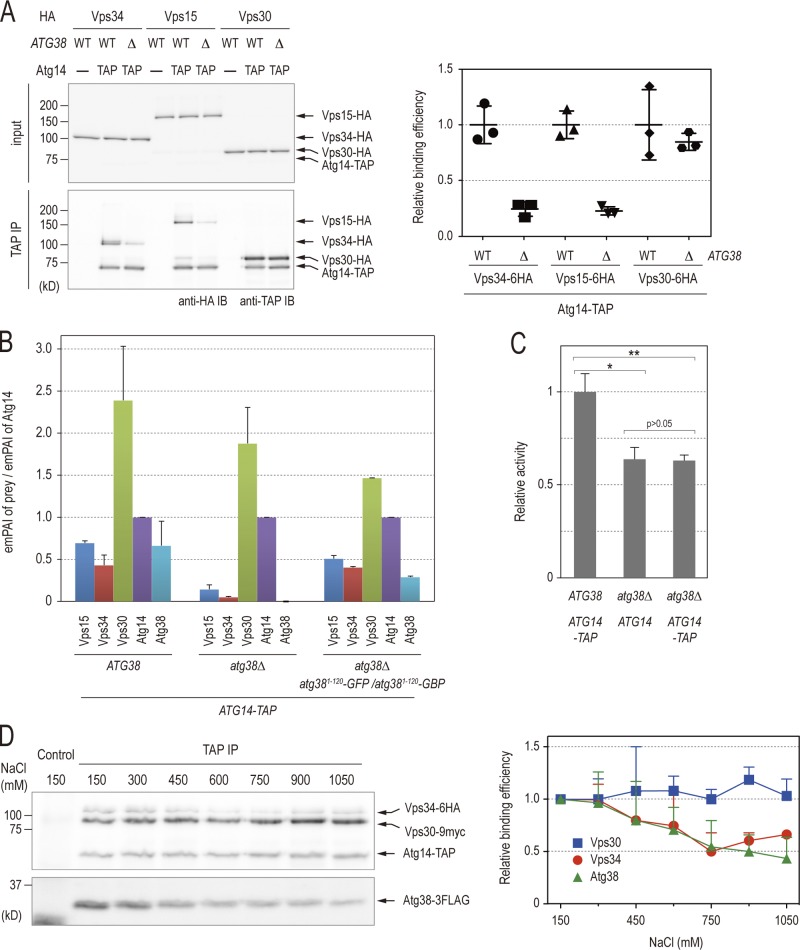
Figure 4.
Atg38 is required to hold the Vps34–Vps15 and Atg14–Vps30 subcomplexes together. (A) Cell extracts were prepared from wild-type and atg38Δ strains expressing TAP-tagged Atg38 and the indicated HA proteins. The extracts were immunoprecipitated by IgG-Dynabeads. The whole-cell extracts (top, “input”) and the precipitates (bottom) were analyzed by immunoblotting with the indicated antibodies. Relative binding efficiency was determined as described in Materials and methods. To compare results from multiple experiments, the value for the reference sample (wild-type cells) was normalized to 1.0. Relative binding efficiency is shown as mean ± SD of three independent experiments. (B) Relative abundances of proteins identified in TAP purifications from the indicated strains were analyzed as described in Fig. 1 C and are shown as mean ± SD of two independent experiments. (C) Cells expressing Pho8Δ60 were grown in YPD and shifted to SD(-N) medium for 3 h at 30°C. Cell lysates from each group of cells were tested for ALP activity. ALP activity is shown as mean ± SD of three independent experiments with the activity of wild-type cells normalized to 1. (D) Cells expressing Vps34-6HA, Vps30-9myc, Atg38-3FLAG, and Atg14-TAP were lysed in a buffer containing 150 mM NaCl. The Atg14-TAP immunoprecipitate with IgG-Dynabeads from this lysate was split into eight fractions and washed with lysis buffer containing the indicated concentrations of NaCl. The resulting precipitates were analyzed by immunoblotting with the indicated antibodies. Relative binding efficiency was determined as described in Materials and methods, although a 150-mM NaCl sample was used as a control and normalized to 1.0. Relative binding efficiency is shown as mean ± SD of three independent experiments.