Abstract
Neurotoxic pesticides, such as neonicotinoids, negatively affect the cognitive capacity and fitness of non-target species, and could also modify interspecific interactions. We tested whether sublethal contamination with neonicotinoid could affect foraging, colony fitness and the outcome of behavioural interactions between a native (Monomorium antarcticum) and an invasive ant species (Linepithema humile). The foraging behaviour of both ants was not affected by neonicotinoid exposure. Colonies of the invasive species exposed to the neonicotinoid produced significantly fewer brood. In interspecific confrontations, individuals of the native species exposed to the neonicotinoid lowered their aggression towards the invasive species, although their survival probability was not affected. Exposed individuals of the invasive species interacting with non-exposed native ants displayed increased aggression and had their survival probability reduced. Non-exposed individuals of the invasive species were less aggressive but more likely to survive when interacting with exposed native ants. These results suggest that non-target exposure of invaders to neonicotinoids could either increase or decrease the probability of survival according to the exposure status of the native species. Given that, in any community, different species have different food preferences, and thus different exposure to pesticides, non-target exposure could potentially change the dynamics of communities and influence invasion success.
Keywords: invasive species, Linepithema humile, Monomorium antarcticum, neonicotinoid, non-target species, sublethal contamination
1. Introduction
Non-target effects of pesticide use are an important global issue. There is increasing evidence that pesticide use at lethal and sublethal concentrations is contributing towards pollinator declines and affecting behavioural responses of non-target organisms [1–4]. Pesticides such as the neonicotinoids are widely used and are effective in the control of many insect pests [5,6]. These chemicals interact with acetylcholine receptors, and directly affect the central and peripheral nervous system of insects [7,8]. Neonicotinoids can impair the cognitive function of insects to such an extent that their ability to interpret external signals and learn is reduced, or even lost, owing to their neurotoxic action [3]. Exposure of pollinators such as bees to pesticides has demonstrated a range of physiological and behavioural changes [8–10]. Bumble-bees exposed to sublethal concentrations of a neonicotinoid had longer foraging trips, decreased food collection and produced fewer workers, and hives had higher worker mortality and loss while foraging [9,10]. Another study showed that small doses of two neonicotinoids (imidacloprid and clothianidin) inhibited the neuronal responses in the brain of honeybees [8], and therefore demonstrated that neonicotinoids act in zones of insect brain responsible for cognition, learning and behaviour.
Sublethal exposure to neonicotinoids may also change the behaviour of other insects. For example, the tunnelling behaviour of the subterranean termite Reticulitermes virginicus reduced when exposed to sublethal doses of the neonicotinoid imidacloprid [11]. Sublethal contamination with imidacloprid also affected the brain development and motility of callow stingless bee workers (Melipona quadrifasciata anthidioides) [12]. The grooming behaviour of the leaf-cutting ant Acromyrmex subterraneus subterraneus, which is a defensive action that prevents colony contamination by pathogens, such as the entomopathogenic fungus Beauveria bassiana, was also reduced after exposure to sublethal doses of imidacloprid [13].
Behaviour also has an important role in determining community shape and dynamics [14]. Changes in behaviour are expected to modulate competitive ability of sympatric and allopatric species, and affect the establishment and spread of newcomers [15]. The success of invasive species, for example, is linked both to their highly aggressive behaviour and to their ability to displace native communities and manipulate food sources [16,17].
In all communities, individual species will have different food preferences. This is certainly the case for ant communities, wherein species are specialized on a particular plant material (e.g. seeds), on indirect consumption of plant material through mutualists (e.g. aphids), or may even be solely predatory in nature [18,19]. Such variation in food preferences probably results in different degrees of exposure to various chemicals, such as pesticides, that may have been released into the environment. Competition for resources substantially influences the success and fitness of many organisms, including social insects such as ants [18]. The ability of ants to compete for resources is frequently related to their colony size and behavioural plasticity [20]. Thus, the modification of behaviour and learning may have broad effects on communities [15]. Changes in behaviours caused by pesticide exposure could moderate the outcome of interspecific interactions. Such changes are probably most relevant when they involve interactions between native and invasive species. Any amplification of the effects of invasive species would be problematic, given their existing role in biodiversity loss and global change [21].
In this study, we exposed colonies of two ant species, the invasive Argentine ant Linepithema humile and the native Southern ant Monomorium antarcticum, to sublethal doses of a neonicotinoid and accounted for the impacts of differential exposure on their interactions and fitness. The Argentine ant is a globally distributed invasive species associated with biodiversity loss and modification [17,22]. In New Zealand, the invasive Argentine ant was first observed in 1990, but is now distributed throughout the North Island and some regions in the South Island [23]. The Southern ant is abundant and widespread within New Zealand [24]. Both species have similar habitat and food preferences, and are aggressive towards each other [20,24], making them an ideal model to evaluate the effects of neonicotinoid pesticides on interacting species. We first assessed the effects of sublethal doses of a neonicotinoid on workers and colonies of each species. Then, we asked whether competitive ability and the outcomes of interspecific interactions between the invasive Argentine ant and the native Southern ant could be influenced by sublethal exposure to the neonicotinoid pesticide.
2. Material and Methods
(a). Colonies and food treatments
Colonies of the Argentine ant and the Southern ant were collected in the field and used in the experiment within seven months of collection. Ant colonies were maintained in laboratory conditions. From the full colonies, we created 10 subcolonies of the native Southern ant and 10 subcolonies of the invasive Argentine ant, each containing 300 workers and two queens. Each subcolony was placed in a plastic container containing three nesting tubes and a segment of plastic tubing connected as a nest exit.
For acclimation to the experimental conditions, colonies were fed for two weeks, three times a week. Food was offered via a cotton pad soaked with 1 ml of a 1 : 4 honey/water (v/v) solution and a mealworm (Tenebrio molitor, larva; approx. 0.09 g) cut into three parts. Five colonies of each species were randomly assigned to each of the treatment groups: colonies treated with sublethal doses of the neonicotinoid imidacloprid (NIC+) (commercial brand: Confidor Yates; active ingredient: imidacloprid 50 g kg−1; water-dispersible granule); and colonies not treated with the neonicotinoid (NIC−). For the NIC+ treatment, 5 ml of honey was mixed with 20 ml of an aqueous solution containing 1.25 µg ml−1 of the neonicotinoid imidacloprid, which resulted in a final concentration of pesticide of 1.0 µg ml−1 administered to the colonies. The use of a low dosage of this insecticide simulates realistic sublethal effects on non-target species [4]. For the NIC− treatment, we offered the honey/water solution with no insecticide.
(b). Experimental design
To assess the effects of sublethal doses of the neonicotinoid on the foraging ability of ant workers and their colony fitness, we conducted trials using a raised wooden maze surrounded by water (see the electronic supplementary material, figure S1). The food resource was randomly assigned to position ‘a’ or ‘b’ on the maze and ants were then allowed to access the maze. During each of the eight trials, which were conducted over 61 days, colonies were observed for a period of 3 h. The following responses were measured: (i) walking speed (how fast an ant crossed a 5 cm-long segment); (ii) food discovery (the time taken for workers to locate the food source); and (iii) drowning rates (the number of ants that fell off the edge of the raised maze into the surrounding water during each trial).
The maze and this experimental set-up simulate habitat complexity and, consequently, factors that could reduce colony fitness if the cognitive system of individuals is affected by sublethal exposure to the neonicotinoid pesticide. In a competitive environment, we expect that changes in walking speed, the probability of food discovery or even the inability of workers to avoid hazards (drowning rates) may compromise their ability to successfully use food sources. Once all trials were fully completed, we quantified the number of live ants remaining and the amount of brood in each colony, which represents an estimate of the effect of the pesticide on the colony fitness.
To assess the effects of sublethal doses of the neonicotinoid on the outcome of interspecific interactions, we randomly selected colonies exposed or not exposed to the pesticide (NIC− or NIC+) and subjected groups to interspecific interactions. We used a 2 × 2 factorial design. Therefore, four different interactive groups were set as follows. Set 1: native (NIC+) versus invasive (NIC+); set 2: native (NIC+) versus invasive (NIC−); set 3: native (NIC−) versus invasive (NIC−); set 4: native (NIC−) versus invasive (NIC+). We also used four external control treatments (n = 10). Controls consisted of groups containing 10 workers subjected to the same colony manipulation procedures and were maintained under the same experimental conditions as the interactive groups. However, control groups were not subjected to interspecific interactions.
Patterns of interspecific interaction were noted as non-aggressive (no harm to the opponent species) or aggressive behaviours (behaviours likely to harm the opponent species). A non-aggressive reaction included the behaviours ignore (body contact with no interest), touch (contact followed by antennation) and avoid (after contact ants retreat in opposite directions). Aggressive responses included the behaviours aggression (head biting, leg biting, raising the gaster or spraying acid) and fight (prolonged aggression—more than 5 s—between individuals; adapted from Suarez et al. [25]). Interspecific interactions were scored for 20 s every 2 min for 20 min (n = 10). Additionally, the number of individuals alive of both species was monitored (n = 20) every 2 min during the first 20 min, then at 25, 30, 40, 50, 60 min, 1, 2, 4, 8, 16 and 32 h.
(c). Statistical analyses
All data analyses and randomizations were performed in R v. 2.15.3 [26]. Ant walking speed and drowning rates were analysed using linear mixed-effect models (LMER) with the package lme4 [27]. Food discovery was analysed using a survival analysis with the package survival [28]. This analysis is appropriate given that data were right-skewed and right-censored. Instead of evaluating the ‘survival probability’, we used the Cox proportional hazard regression models (Coxph) to assess the effects of neonicotinoid exposure on the probability of ants to find the food source. The number of workers alive and the quantity of brood after 61 days of trials were compared using generalized linear models (GLM) with a Gaussian family distribution.
Interspecific interaction level between groups of workers in different treatments was analysed using generalized mixed-effect models (GLMER) with the package lme4 [27]. The two behavioural reactions (non-aggressive or aggressive) were modelled as a binary response. The survival probability of ants in different treatments was also analysed using the package survival [28]. We used Cox proportional hazard regression models to compare the survival probability of interacting groups in different treatments, including controls. For all randomizations, we used the function sample(). Significance for all tests was assumed at p < 0.05.
Further details of methods, including a description of the experimental set-up, insecticide preparation, feeding treatments, experimental design and additional information regarding the statistical analyses, are available in the electronic supplementary material.
3. Results
(a). Effects of sublethal doses of the neonicotinoid on workers and colony fitness
The walking speed and drowning rate of the native Southern ant and the invasive Argentine ant were not significantly affected by exposure to the neonicotinoid (table 1; p ≥ 0.051). Although the invasive Argentine ant was more likely to find food sources (figure 1; d.f. = 3, b = −0.83, z = −3.18, p = 0.002, Coxph), the food discovery probability of both the native Southern ant (d.f. = 3, b = −0.021, z = −0.075, p = 0.940, Coxph) and invasive Argentine ant (d.f. = 3, b = 0.168, z = 0.727, p = 0.467, Coxph) were not affected by exposure to the neonicotinoid.
Table 1.
Mean (± s.e.) and the linear mixed-effect model results comparing the walking speed and drowning rate of ants from colonies not exposed (NIC−) and exposed (NIC+) to sublethal doses of the neonicotinoid. For each treatment, n = 40.
| species | response | mean (±s.e.) |
b (s.e.) | t | p | |
|---|---|---|---|---|---|---|
| NIC− | NIC+ | |||||
| native Southern ant | walking speed (cm s−1) drowning rate | 0.455 (−0.020) 2.854 (0.448) | 0.476 (−0.015) 2.038 (0.315) | 0.021 (0.06) −0.816 (0.55) | 0.371 −1.490 | 0.712 0.140 |
| invasive Argentine ant | walking speed (cm s−1) drowning rate | 0.833 (0.028) 1.137 (0.176) | 0.765 (0.021) 2.246 (0.446) | −0.068 (0.03) 1.109 (0.75) | −1.980 1.473 | 0.051 0.145 |
Figure 1.
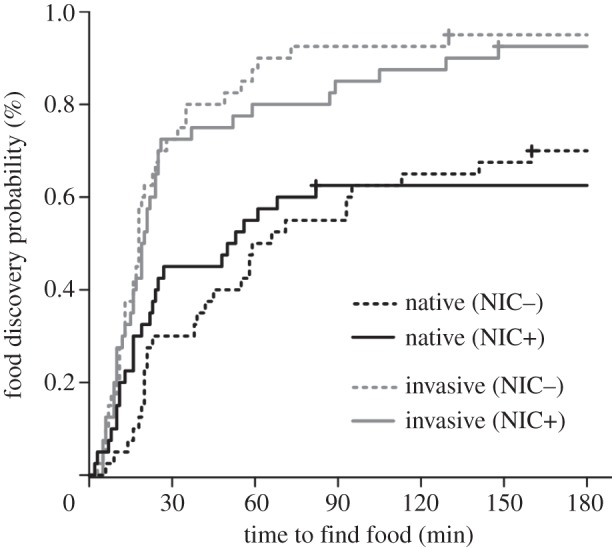
The food discovery probability over time for the native Southern ant (black lines) and the invasive Argentine ant (grey lines). Dashed lines: colonies not exposed to the neonicotinoid (NIC−); solid lines: colonies exposed to sublethal doses of the neonicotinoid (NIC+). The food discovery probability is a one minus transformation of the estimated Kaplan–Meier probability curves. For each treatment, n = 40.
There were no differences in the number of workers alive after 61 days of trials in colonies of either the native Southern ant or the invasive Argentine ant (figure 2a; d.f. = 1, d.f.error = 17, b = −18.5, t = −0.67, p = 0.512, GLM), regardless of treatment (d.f. = 1, d.f.error = 17, b = −49.7, t = −1.8, p = 0.089, GLM). However, the effects that neonicotinoid exposure had on brood production (figure 2b) differed between the native Southern ant and the invasive Argentine ant (d.f. = 1, d.f.error = 16, b = −173.4, t = −3.19, p = 0.006, GLM). Although brood production of the native Southern ant was not affected by sublethal doses of the neonicotinoid (d.f. = 1, d.f.error = 16, b = −1.5, t = −0.06, p = 0.957, GLM), the brood production of the invasive Argentine ant was reduced in the NIC+ treatment (d.f. = 1, d.f.error = 16, b = −151.4, t = −2.79, p = 0.013, GLM).
Figure 2.
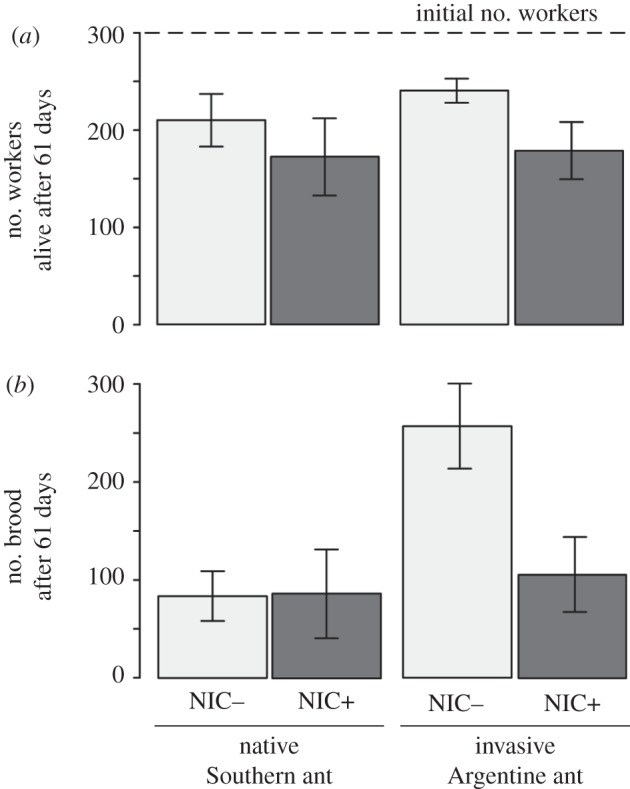
The number of (a) workers alive and (b) brood produced on colonies of the native Southern ant and the invasive Argentine ant after 61 days of trials (mean ± s.e.). Light grey columns: colonies of both species not exposed to the neonicotinoid (NIC−). Dark grey columns: colonies of both species exposed to sublethal doses of the neonicotinoid (NIC+). The dashed line in (a) is the initial number of workers in each colony for all treatments. For each treatment, n = 5.
(b). Effects of sublethal doses of the neonicotinoid on interspecific interaction and survival probability
Groups of 10 workers from both exposed and non-exposed colonies were subjected to interspecific interactions. During interspecific interactions, the native Southern ant and the invasive Argentine ant displayed higher rates of aggressive behaviours than non-aggressive behaviours, regardless of their treatment status (figure 3; native: d.f. = 1, b = −1.53, z = −9.69, p < 0.001; invasive: d.f. = 1, b = −0.78, z = −6.43, p < 0.001, GLMER). The aggressive behaviour of the native Southern ant (figure 3a) was significantly lower when exposed to the neonicotinoid (d.f. = 1, b = −0.41, z = −4.49, p < 0.001, GLMER), regardless of the treatment status of the invasive Argentine ant (d.f. = 1, b = 0.11, z = 1.18, p = 0.239, GLMER).
Figure 3.
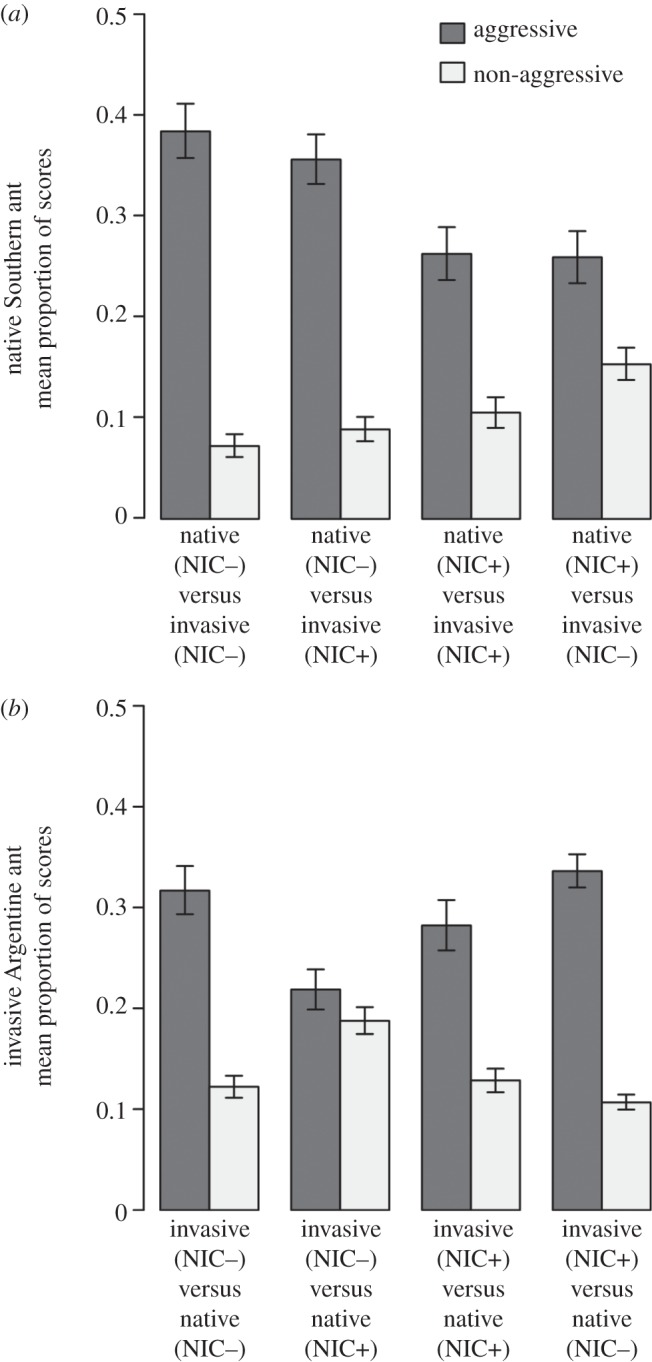
The proportion (mean ± s.e.) of behavioural reactions displayed between groups containing 10 workers of each ant species in different treatment statuses. Groups of interacting ants were not exposed (NIC−) or exposed (NIC+) to sublethal doses of the neonicotinoid. Dark grey columns are aggressive responses. Light grey columns are non-aggressive responses. (a) The proportion of responses displayed by the native Southern ant during interactions with the invasive Argentine ant. (b) The proportion of responses displayed by the invasive Argentine ant during interactions with the native Southern ant. For each treatment, n = 10.
The aggressive behaviour of the invasive Argentine ant (figure 3b) was affected by both their treatment status (d.f. = 1, b = 0.31, z = 3.91, p < 0.001, GLMER) and the treatment status of the native Southern ant (d.f. = 1, b = −0.42, z = −5.39, p < 0.001, GLMER). Interestingly, the invasive Argentine ant did not modify their aggressive response towards the native Southern ant when both were exposed to the neonicotinoid (NIC+ versus NIC+) and under standard conditions (NIC− versus NIC−; d.f. = 1, b = −0.12, z = −1.02, p = 0.308, GLMER). Even though the invasive Argentine ant was not exposed to the neonicotinoid in the ‘invasive (NIC−) versus native (NIC+)’ treatment, they became less aggressive towards groups of exposed native Southern ant (d.f. = 1, b = −0.35, z = 3.15; p = 0.002, GLMER). Conversely, in the ‘invasive (NIC+) versus native (NIC−)’ treatment the invasive Argentine ant displayed the highest levels of aggression (d.f. = 1, b = −0.47, z = −3.57, p < 0.001, GLMER).
After 32 h of interspecific interaction, the survival probability of the native Southern ant (figure 4a) was not affected in any treatment regardless of their treatment status (d.f. = 3, b = 0.02, z = 0.93, p = 0.351, Coxph) or the treatment status of the invasive Argentine ant (d.f. = 3, b = −0.04, z = −1.46, p = 0.144, Coxph). Furthermore, the external control groups of the native Southern ant, not subjected to interspecific interactions, did not differ from the other groups subjected to interspecific interaction with the invasive Argentine ant (d.f. = 3, b = −0.02, z = −0.01, p = 0.994, Coxph).
Figure 4.
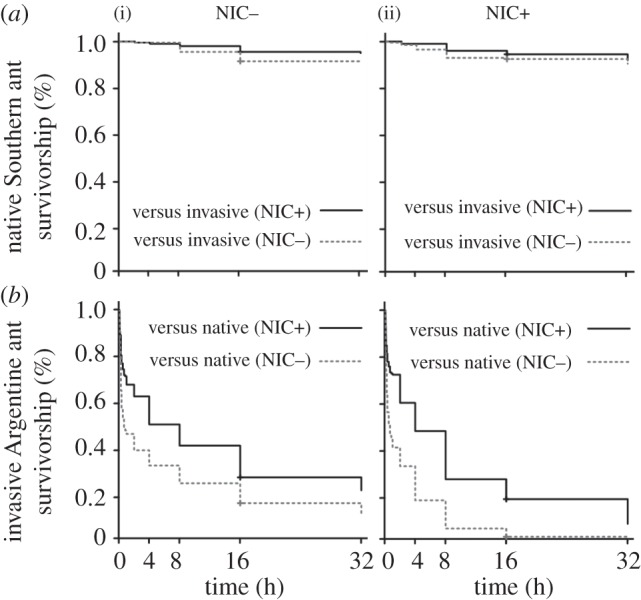
The survival probability of ants subjected to interspecific interactions in groups containing 10 workers of each ant species in different treatment statuses. Groups of interacting ants were not exposed (NIC−) or exposed (NIC+) to sublethal doses of the neonicotinoid. (a) Survival probability over time of the native Southern ant during interactions with the invasive Argentine ant. (b) Survival probability over time of the invasive Argentine ant during interactions with the native Southern ant. For each treatment, n = 20.
The survival probability of the invasive Argentine ant (figure 4b) was influenced by both their treatment status (d.f. = 3, b = 0.53, z = 5.06, p < 0.001, Coxph) and the treatment status of the native Southern ant (d.f. = 3, b = −0.46, z = −4.13, p < 0.001, Coxph). The survival probability of the invasive Argentine ant in natural conditions (NIC− versus NIC−) was relatively low (figure 4b), and did not change when both the native Southern ant and Argentine ant were exposed to the pesticide (NIC+ versus NIC+; d.f. = 3, b = 0.8, z = −1.48, p = 0.14, Coxph). Surprisingly, in the ‘invasive (NIC+) versus native (NIC−)’ treatment, in which the invasive Argentine ant displayed the highest levels of aggression (figure 3b), the invasive Argentine ant was completely exterminated during the first 16 h of interaction (d.f. = 3, b = 0.68, z = 6.54, p < 0.001, Coxph). Importantly, in the ‘invasive (NIC−) versus native (NIC+)’ treatment, in which the invasive Argentine ant displayed the lowest aggression levels (figure 3b), we found significant increased survival probability of the invasive Argentine ant (d.f. = 3, b = −0.31, z = −2.83, p = 0.005, Coxph). Groups of the invasive Argentine ant subjected to interspecific interaction had lower survival probability than their external control groups not subjected to interactions (d.f. = 3, b = −3.09, z = −9.92, p < 0.001, Coxph).
4. Discussion
We found that exposure to neonicotinoids can alter the behaviour, fitness and community dynamics of ants. Our experiment demonstrates that exposure to neonicotinoids had different impacts on the interspecific aggressive behaviour and colony fitness of the native Southern ant and the invasive Argentine ant. The invasive Argentine ant, whether exposed or not exposed to the neonicotinoid, presented higher ability to locate and explore food sources than the native Southern ant. Brood production of the native Southern ant was not affected by the neonicotinoid. However, an important effect of sublethal exposure to the neonicotinoid in the invasive Argentine ant was to reduce brood numbers to approximately 50% of those in non-exposed colonies.
The success of the invasive Argentine ant is partially linked to their rapid recruitment and dominance of food sources [17]. While neonicotinoids modified the foraging ability of bees [9], we found no effects of sublethal exposure on the foraging ability of either the native Southern ant or the invasive Argentine ant. Bees and ants use different cues to locate and inform food position. Most ants use species- or colony-specific pheromones to guide themselves and recruit nest-mates to food sources. Chemosensory receptors located on their antennae identify the odour produced by colony members [29]. On the other hand, foraging activity and orientation in bees is coordinated by ritualized modes of communication, including the waggle dance [30]. Different species within any community are likely to use different methods to perceive food resources or potential hazards [15]. We observed no such effects in our system. Because neonicotinoids affect specific neuronal pathways, and consequently behaviours, we expected variation in neonicotinoid effects between species.
In a first scenario, where both the native Southern ant and invasive Argentine ant were exposed or not exposed to the neonicotinoid, we found no significant effects of the pesticide on the aggression level and survival probability of the invasive species. A second scenario where only the invasive Argentine ant was exposed to neonicotinoid prior to interaction with the native Southern ant showed that the invasive Argentine ant displayed higher levels of aggression, but was completely exterminated by the native Southern ant. Importantly, in a third scenario where only the native Southern ant was exposed to the pesticide prior to interaction with the invasive Argentine ant, we found that the invasive Argentine ants reduced their aggression but had increased survival probability.
The distribution of the invasive Argentine ant throughout the world is strongly linked with anthropogenic activities and also, to a smaller degree, with their biotic interaction with local species [22]. In New Zealand, for instance, the invasive Argentine ant is only found in urban and agricultural settings co-occurring (and possibly competing for food sources) with the native Southern ant [20,23]. In these areas, pesticides such as neonicotinoids are commonly used to control insect pests and may affect ants via distinct pathways such as direct contact with the active ingredient applied in the environment, consumption of plant material containing the pesticide or even ingestion of honeydew produced by mutualists. Other ant species in these communities are, for example, solely predatory in nature, just as in any other community. Thus, differential exposure to pesticides would almost certainly occur between species within any community.
It is important to highlight possible non-target effects of neonicotinoids on the biotic resistance imposed by native communities. In any given habitat where the local species had been previously exposed to neonicotinoids, the invasive Argentine ant could have significantly higher chances to monopolize food sources and survive. The reduced brood production of Argentine ant colonies exposed to the neonicotinoid gave them a similar outcome to the native Southern ant. Hence, in areas where both the native Southern ant and invasive Argentine ant co-occur, it is likely that the reduction in brood production as a result of sublethal exposure to the neonicotinoid in the invasive Argentine ant is more significant to colony survival than the behavioural responses.
We note that there may be important effects of neonicotinoids on the invasive Argentine ant, depending on the community composition and context. In areas extensively dominated by the invasive Argentine ant, the combined effects of target and non-target pest control programmes may exert synergistic effects and improve their control [31]. Herein, for example, groups of Argentine ants previously exposed to the neonicotinoid had reduced brood production and were annihilated by groups of the native Southern ant that were not exposed to the pesticide. The reduced brood production of the invasive Argentine ant may significantly affect recently established colonies, in which the number of queens and workers are relatively small [32]. These non-target effects combined with an appropriate control programme targeting Argentine ants [33] could efficiently suppress their population in invaded areas.
The role of behaviour in determining the success of species and shaping communities is well established [14,18]. The dose-dependent impacts of neonicotinoids in the neuronal activity of insect brains could impair cognition and learning of new behavioural tasks [8]. Our results showed that neonicotinoids affect behaviour and fitness of different species in different ways. Thus, non-target effects of this neurotoxic pesticide could potentially have detrimental effects on natural communities and act as a human-mediated driver of invasion. Uncontrolled use of neonicotinoids in urban and agricultural areas could modify aggressive responses and the outcome of interspecific interactions. Our results provide evidence of the potential effects of pesticides on the structure and dynamics of ant communities. We believe that, within any community, different food preferences and behaviours between species will result in differential exposure to pesticides such as neonicotinoids. This exposure can clearly alter both intraspecific behaviours and the outcome of interactions within the community.
Acknowledgements
We are grateful to Victoria University of Wellington for granting a PhD scholarship to R.F.B. and to the Centre for Ecology and Biodiscovery for granting a summer scholarship to A.S.M. We also thank Alexander Stainton, Ford Anderson, Gabi Hidvegi, Heather Barnes and James Wilson for their engagement on the pilot experiment of this research as part of the Global Change Biology course (School of Biological Sciences, Victoria University of Wellington). Thanks are also extended to Deborah Gordon and the anonymous reviewers for insightful comments on this manuscript.
References
- 1.Desneux N, Decourtye A, Delpuech JM. 2007. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 52, 81–106 (doi:10.1146/Annurev.Ento.52.110405.091440) [DOI] [PubMed] [Google Scholar]
- 2.Johnson RM, Ellis MD, Mullin CA, Frazier M. 2010. Pesticides and honey bee toxicity—USA. Apidologie 41, 312–331 (doi:10.1051/Apido/2010018) [Google Scholar]
- 3.Williamson SM, Wright GA. 2013. Exposure to multiple cholinergic pesticides impairs olfactory learning and memory in honeybees. J. Exp. Biol. 216, 1799–1807 (doi:10.1242/jeb.083931) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blacquiere T, Smagghe G, van Gestel CAM, Mommaerts V. 2012. Neonicotinoids in bees: a review on concentrations, side-effects and risk assessment. Ecotoxicology 21, 973–992 (doi:10.1007/S10646-012-0863-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomizawa M, Casida JE. 2005. Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu. Rev. Pharmacol. Toxicol. 45, 247–268 (doi:10.1146/annurev.pharmtox.45.120403.095930) [DOI] [PubMed] [Google Scholar]
- 6.Watson GB, Loso MR, Babcock JM, Hasler JM, Letherer TJ, Young CD, Zhu YM, Casida JE, Sparks TC. 2011. Novel nicotinic action of the sulfoximine insecticide sulfoxaflor. Insect Biochem. Mol. Biol. 41, 432–439 (doi:10.1016/j.ibmb.2011.01.009) [DOI] [PubMed] [Google Scholar]
- 7.Brown LA, Ihara M, Buckingham SD, Matsuda K, Sattelle DB. 2006. Neonicotinoid insecticides display partial and super agonist actions on native insect nicotinic acetylcholine receptors. J. Neurochem. 99, 608–615 (doi:10.1111/J.1471-4159.2006.04084.X) [DOI] [PubMed] [Google Scholar]
- 8.Palmer MJ, Moffat C, Saranzewa N, Harvey J, Wright GA, Connolly CN. 2013. Cholinergic pesticides cause mushroom body neuronal inactivation in honeybees. Nat. Commun. 4, 1634 (doi:10.1038/ncomms2648) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gill RJ, Ramos-Rodriguez O, Raine NE. 2012. Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature 491, U105–U119 (doi:10.1038/Nature11585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitehorn PR, O'Connor S, Wackers FL, Goulson D. 2012. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336, 351–352 (doi:10.1126/Science.1215025) [DOI] [PubMed] [Google Scholar]
- 11.Thorne BL, Breisch NL. 2001. Effects of sublethal exposure to imidacloprid on subsequent behavior of subterranean termite Reticulitermes virginicus (Isoptera: Rhinotermitidae). J. Econ. Entomol. 94, 492–498 (doi:10.1603/0022-0493-94.2.492) [DOI] [PubMed] [Google Scholar]
- 12.Tome HVV, Martins GF, Lima MAP, Campos LAO, Guedes RNC. 2012. Imidacloprid-induced impairment of mushroom bodies and behavior of the native stingless bee Melipona quadrifasciata anthidioides. PLoS ONE 7, e38406 (doi:10.1371/journal.pone.0038406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galvanho JP, Carrera MP, Moreira DDO, Erthal M, Silva CP, Samuels RI. 2013. Imidacloprid inhibits behavioral defences of the leaf-cutting ant Acromyrmex subterraneus subterraneus (Hymenoptera: Formicidae). J. Insect Behav. 26, 1–13 (doi:10.1007/S10905-012-9328-6) [Google Scholar]
- 14.Sih A, Cote J, Evans M, Fogarty S, Pruitt J. 2012. Ecological implications of behavioural syndromes. Ecol. Lett. 15, 278–289 (doi:10.1111/J.1461-0248.2011.01731.X) [DOI] [PubMed] [Google Scholar]
- 15.Wolf M, Weissing FJ. 2012. Animal personalities: consequences for ecology and evolution. Trends Ecol. Evol. 27, 452–461 (doi:10.1016/j.tree.2012.05.001) [DOI] [PubMed] [Google Scholar]
- 16.Holway DA, Suarez AV. 1999. Animal behavior: an essential component of invasion biology. Trends Ecol. Evol. 14, 328–330 (doi:10.1016/S0169-5347(99)01636-5) [DOI] [PubMed] [Google Scholar]
- 17.Holway DA, Lach L, Suarez AV, Tsutsui ND, Case TJ. 2002. The causes and consequences of ant invasions. Annu. Rev. Ecol. Syst. 33, 181–233 (doi:10.1146/Annurev.Ecolysis.33.010802.150444) [Google Scholar]
- 18.Cerdá X, Arnan X, Retan J. 2013. Is competition a significant hallmark of ant (Hymenoptera: Formicidae) ecology? Myrmecol. News 18, 131–147 [Google Scholar]
- 19.Lester PJ, Baring CW, Longson CG, Hartley S. 2003. Argentine and other ants (Hymenoptera: Formicidae) in New Zealand horticultural ecosystems: distribution, hemipteran hosts, and review. N. Z. Entomol. 26, 79–89 (doi:10.1080/00779962.2003.9722112) [Google Scholar]
- 20.Sagata K, Lester PJ. 2009. Behavioural plasticity associated with propagule size, resources, and the invasion success of the Argentine ant Linepithema humile. J. Appl. Ecol. 46, 19–27 (doi:10.1111/J.1365-2664.2008.01523.X) [Google Scholar]
- 21.Brook BW, Sodhi NS, Bradshaw CJA. 2008. Synergies among extinction drivers under global change. Trends. Ecol. Evol. 23, 453–460 (doi:10.1016/J.Tree.2008.03.011) [DOI] [PubMed] [Google Scholar]
- 22.Roura-Pascual N, et al. 2011. Relative roles of climatic suitability and anthropogenic influence in determining the pattern of spread in a global invader. Proc. Natl Acad. Sci. USA 108, 220–225 (doi:10.1073/Pnas.1011723108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ward DF, Green C, Harris RJ, Hartley S, Lester PJ, Stanley MC, Suckling DM, Toft RJ. 2010. Twenty years of Argentine ants in New Zealand: past research and future priorities for applied management. N. Z. Entomol. 33, 68–78 (doi:10.1080/00779962.2010.9722193) [Google Scholar]
- 24.Don W. 2007. Ants of New Zealand, p. 239 Dunedin, New Zealand: Otago University Press [Google Scholar]
- 25.Suarez AV, Tsutsui ND, Holway DA, Case TJ. 1999. Behavioral and genetic differentiation between native and introduced populations of the Argentine ant. Biol. Invasions 1, 43–53 (doi:10.1023/A:1010038413690) [Google Scholar]
- 26.R Development Core Team 2013. R: a language and environment for statistical computing. 2.15.3 edn. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 27.Bates D, Maechler M, Bolker B.2012. lme4: linear mixed-effects models using S4 classes. R package version 0.999999-0. See http://CRAN.R-project.org/package=lme4.
- 28.Therneau T.2012. A package for survival analysis in S. R package version 2.37–2. See http://CRAN.R-project.org/package=survival.
- 29.Ozaki M, Wada-Katsumata A, Fujikawa K, Iwasaki M, Yokohari F, Satoji Y, Nisimura T, Yamaoka R. 2005. Ant nestmate and non-nestmate discrimination by a chemosensory sensillum. Science 309, 311–314 (doi:10.1126/Science.1105244) [DOI] [PubMed] [Google Scholar]
- 30.Riley JR, Greggers U, Smith AD, Reynolds DR, Menzel R. 2005. The flight paths of honeybees recruited by the waggle dance. Nature 435, 205–207 (doi:10.1038/Nature03526) [DOI] [PubMed] [Google Scholar]
- 31.Brightwell RJ, Bambara SB, Silverman J. 2010. Combined effect of hemipteran control and liquid bait on Argentine ant populations. J. Econ. Entomol. 103, 1790–1796 (doi:10.1603/ec10150) [DOI] [PubMed] [Google Scholar]
- 32.Silverman J, Brightwell RJ. 2008. The Argentine ant: challenges in managing an invasive unicolonial pest. Annu. Rev. Entomol. 53, 231–252 (doi:10.1146/annurev.ento.53.103106.093450) [DOI] [PubMed] [Google Scholar]
- 33.Rust MK, Reierson DA, Klotz JH. 2004. Delayed toxicity as a critical factor in the efficacy of aqueous baits for controlling argentine ants (Hymenoptera: Formicidae). J. Econ. Entomol. 97, 1017–1024 (doi:10.1603/0022-0493(2004)097[1017:DTAACF]2.0.CO;2) [DOI] [PubMed] [Google Scholar]


