Abstract
Animals can use different sources of information when making decisions. Foraging animals often have access to both self-acquired and socially acquired information about prey. The fringe-lipped bat, Trachops cirrhosus, hunts frogs by approaching the calls that frogs produce to attract mates. We examined how the reliability of self-acquired prey cues affects social learning of novel prey cues. We trained bats to associate an artificial acoustic cue (mobile phone ringtone) with food rewards. Bats were assigned to treatments in which the trained cue was either an unreliable indicator of reward (rewarded 50% of the presentations) or a reliable indicator (rewarded 100% of the presentations), and they were exposed to a conspecific tutor foraging on a reliable (rewarded 100%) novel cue or to the novel cue with no tutor. Bats whose trained cue was unreliable and who had a tutor were significantly more likely to preferentially approach the novel cue when compared with bats whose trained cue was reliable, and to bats that had no tutor. Reliability of self-acquired prey cues therefore affects social learning of novel prey cues by frog-eating bats. Examining when animals use social information to learn about novel prey is key to understanding the social transmission of foraging innovations.
Keywords: bat, novel prey, social information, social learning, Trachops cirrhosus
1. Introduction
Social information, or information acquired from others, is used by a wide variety of taxa in behavioural contexts that range from foraging (e.g. [1–3]) to mate choice (e.g. [4–7]). Social learning is an efficient way to acquire information because it avoids costly mistakes that can be made during trial-and-error learning. Social information, however, can have its own costs in terms of outdated or inaccurate information, or costs associated with interactions with conspecifics [8,9]. These costs and benefits of social information have led to the prediction that animals should use social information selectively in combination with self-acquired information, following particular ‘social learning strategies’ [9–12]. Extensive research in fishes has provided important insights into the role of social information in decisions about where to find food (reviewed in [9,12,13]). Less is known about social learning strategies in animals that use social information to learn novel cues that indicate prey.
Owing to the risks of consuming unpalatable food, acquiring information about novel food is suggested as one of the key advantages of social learning [2]. Social learning of novel food has been demonstrated for a number of species (e.g. [14–16]). To understand how behavioural innovations might spread through natural populations, it is crucial to examine not only whether animals are capable of using social information to learn novel behaviours, but also when animals are likely to use that social information. This has been most thoroughly studied in Norway rats, in which naive observers acquire information about novel food from the breath of experienced conspecifics [17]. Satisfaction, uncertainty, predation risk and environmental stability affect the use of social information in rats (reviewed in [18]). This research is key to predicting how and when food preferences are transmitted in groups of rats. Addressing similar questions in non-model systems is important in order to understand the generalizability of social learning strategies. Here, we examined social learning strategies in wild-caught frog-eating bats that can use social information to learn novel acoustic prey cues.
The availability of foraging information has been proposed as an advantage leading to the evolution of bat coloniality [19], and a number of studies have demonstrated social learning in bats [20–23]. The fringe-lipped bat, Trachops cirrhosus, is a Neotropical carnivore that hunts frog and insect prey by eavesdropping on the prey's mating calls [24,25]. Trachops cirrhosus differentiates poisonous and palatable frog species by their calls [24], but is quite flexible in these associations and bats can be trained to reverse their preferences [26]. Additionally, T. cirrhosus can learn novel associations between prey cue and prey quality by observing foraging conspecifics [22]. In this study, we examined how the reliability of self-acquired cues about prey influences the use of social information to learn novel prey cues.
We created in captivity a scenario in which bats that are foraging on a self-acquired prey cue can interact with a conspecific that is approaching a different, novel, prey cue. We wished to examine how the reliability with which bats received rewards when they approached self-acquired cues affects the use of social information to learn novel prey cues. We used reward schedule (here termed ‘reliability’) as a proxy for natural capture success. Our prediction was that bats with 50% reliable self-acquired prey cues would be more likely to use social information to learn novel prey cues than bats with 100% reliable self-acquired prey cues. Different prey species emit different acoustic cues, but they may also be associated with particular calling sites. Trachops cirrhosus could learn to approach novel prey by observing a conspecific approach a specific prey cue, by observing the bat's approach to a particular location or by a combination of these mechanisms. We therefore also determined whether bats are more likely to learn to approach the location from which the novel cue was broadcast or the cue itself.
2. Material and methods
(a). Experimental animals and arena
We captured bats (n = 18 adult males) between February and December 2011 in Soberanía National Park, Panamá. Experiments were conducted in a 5 × 5 × 2.5 m flight cage under ambient temperature and humidity, illuminated by a 25 W red light bulb. We placed Fostex FE103En speakers underneath 1.5 × 1.5 m screens covered in leaf-litter in two corners of the cage. In the third corner was a shelter to which the bat was trained to return between cue presentations; the experimenter sat in the final corner opposite the shelter with the sound playback and video recording equipment. Sound playback was conducted through a Pyle Pro PTA2 amplifier and a Lenovo T500 Thinkpad laptop. We used two Sony Handycam DCR-SR45 digital video camera recorders and additional Sony HVL-IRM infrared lights to record responses. Before testing, bats that were housed and tested together were given individual-specific haircuts to enable experimenter recognition. After testing, all bats were individually marked with passive integrated transponder tags (Trovan Ltd.) and released at their capture sites.
(b). Experimental overview
The experiment consisted of five components: the initial training phase (at least 40 trials), a set of pre-tests (six trials), an experimental exposure phase (100 trials), a set of post-tests (six trials) and a set of cue/location tests (four trials). A flow chart summarizes the order of the experimental components (figure 1), while the subsections below detail the methods used in each individual component.
Figure 1.
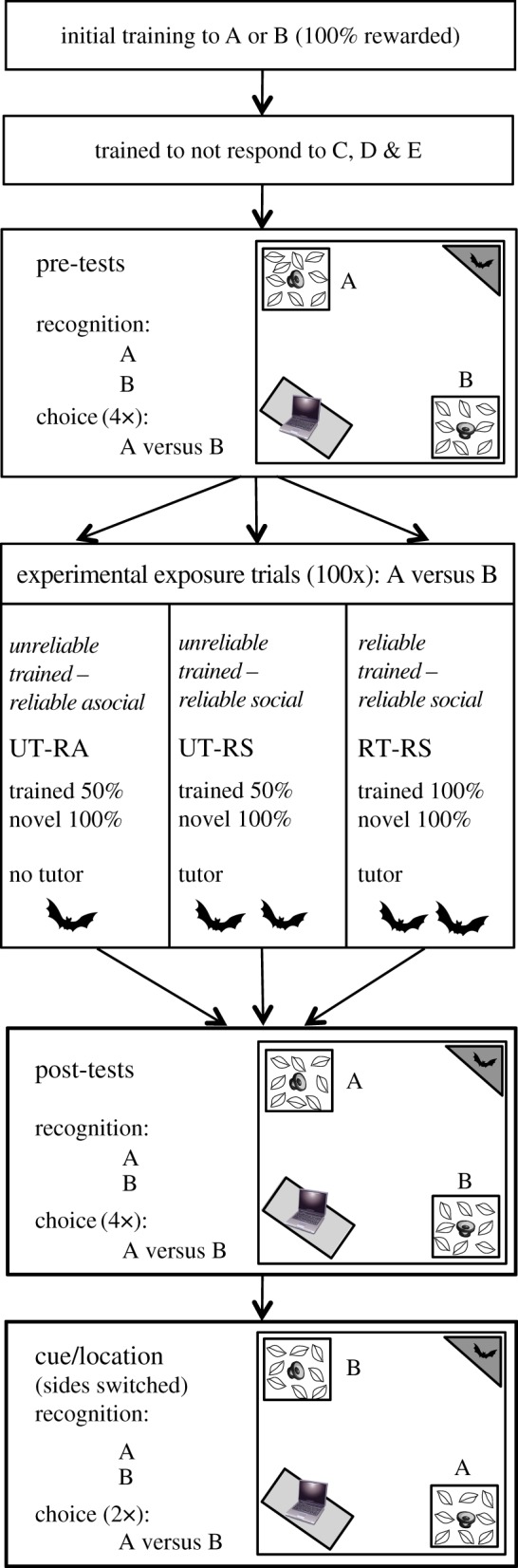
Protocol overview. Flight cage diagram is not to scale.
(c). Initial training
We trained bats to associate one of two mobile phone ringtones (A or B, figure 2; electronic supplementary material, videos) with food rewards. Ringtones were of approximately the same duration (0.6 s) and dominant frequency (750 Hz) as the call of the túngara frog, Physalaemus (=Engystomops) pustulosus, a preferred prey species of this bat [24], but sounded very different to human ears. We used ringtones to ensure bats had no previous associations with experimental cues. To train bats to approach the ringtones, we created stimuli in which we merged the túngara frog call and ringtones using Adobe Audition v. 3.0, and adjusted the relative RMS amplitudes to fade out the frog call and fade in the ringtone in five steps [26]. Food rewards consisted of small pieces of baitfish placed on the speaker for each stimulus presentation. Training was completed in one to two nights, and the number of trials required (mean trials ± s.e.: A = 11.3 ± 2.6, B = 13.0 ± 2.1) did not significantly differ between the two cues (Welch two-sample t-test: t = −0.5, d.f. = 12.8, p = 0.6). To ensure that each bat had ample experience with the trained cue, they were presented with the cue associated with a food reward at least 40 times (mean ± s.e. = 59.3 ± 4.0 trials) before advancing to the pre-tests. During this training period, the cue was rewarded every presentation and broadcast alternately from the two speakers to ensure that bats did not develop a location preference.
Figure 2.
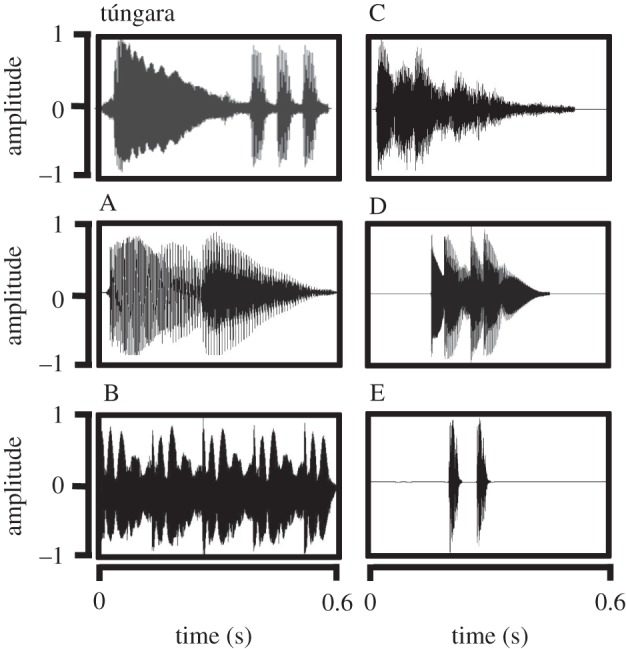
Waveforms of experimental stimuli: the túngara frog call used for training, experimental ringtones/cues A and B, and extinguishing ringtones C, D and E.
Frog-eating bats have been documented to generalize their responses to known stimuli to respond to novel stimuli [27]. To ensure that bats were selectively approaching their trained cue and not all acoustic stimuli, we interspersed presentations of the trained cue with presentations of other ringtones (C, D and E, figure 2) that were never associated with food rewards. Generalization was extinguished rapidly with these unrewarded trials (mean number of presentations required for extinction of generalized response ± s.e.: C = 2.7 ± 0.9, D = 2.3 ± 0.8, E = 3.3 ± 1.4). The number of extinction trials required did not differ significantly between ringtones (negative binomial GLM: χ2 = 0.54, d.f. = 2, p = 0.76). We consistently rewarded the trained cue in these initial training trials to facilitate specific associative learning of the trained stimulus and reduce generalization to the other stimuli (C, D and E).
(d). Pre-tests
After training, bats in all treatments were given identical pre-tests to establish a baseline of their responses to the trained cue and the ringtone that would be the novel cue for the experimental exposure trials (A if the trained cue was B or vice versa). Pre-tests were composed of two single-speaker recognition tests (one for A and one for B) and four two-speaker preference tests (A versus B). Recognition tests determined whether bats responded to the cue; each consisted of one ringtone broadcast from a single speaker 10 times with a 1 s interval of silence between each ringtone, or until the bat landed on the speaker. Preference tests assessed which of the two cues the bat preferred, and consisted of presentations of A and B antiphonally from two speakers in opposite corners of the arena (approx. 6 m apart) 10 times or until the bat landed on one of the speakers [26]. Pre-tests were rewarded with baitfish placed on both speakers.
(e). Experimental exposure
For the experimental exposure trials, focal bats were randomly assigned to one of three treatments that varied in the reliability (reward schedule) of the trained cue and the presence of a tutor (n = 6 bats per treatment). The novel cue was always reliably (100%) associated with food rewards. In the unreliable trained–reliable asocial (UT-RA) treatment, the trained cue was 50% rewarded (every other presentation) and the novel cue was broadcast with food rewards placed on the speaker for 100% of the presentations but there was no tutor bat present. In the unreliable trained–reliable social (UT-RS) treatment, the cue to which the bats were trained was rewarded 50% of the time (every other presentation) and there was a tutor bat foraging on the reliable novel cue. In the reliable trained–reliable social (RT-RS) treatment, the focal bats had a reliable trained cue (maintained 100% rewarded) and they were exposed to a tutor foraging on the reliable novel cue (figure 1). Whenever the cue playback was rewarded multiple small food rewards were placed on the speaker. If both the focal and tutor bat approached the stimulus, they both had an opportunity to get a food reward, thereby reducing the likelihood of any competition between the two bats.
In the experimental exposure trials, stimuli A and B were broadcast antiphonally from the two speakers 10 times each or until one of the bats landed on a speaker. One or both of the two bats (focal bat and tutor) had to approach a speaker in order to proceed to the next trial. The 100 experimental exposure trials required four to five nights to complete. During this time, the tutor and focal bat were housed together. We recorded which cue the focal bat approached for each trial. We compared the number of focal bats in each treatment that approached the novel cue during the experimental exposure trials with Fisher's exact tests in R version 2.15 [28]. To examine the effect of treatment on the number of trials required for focal bats to approach the novel cue, we conducted negative binomial generalized estimating equations (GEEs, GLM) using the R MASS package [29].
(f). Post-tests
After the 100 experimental exposure trials, the tutor was removed (if present) and the focal bat was given post-tests that were identical to the pre-tests (four preference tests and two recognition tests). We compared the effect of treatment on preference for the novel cue (clustered by individual bat) with a binomial GEE using the R geepack package [30]. We also examined the effect of treatment on the number of bats that approached each cue in the recognition tests using Fisher's exact tests.
(g). Cue/location tests
The sides of the arena from which the trained and novel cues were broadcast were maintained for the pre-tests, experimental exposure trials and post-tests. This was a precaution to ensure that the tutor bat consistently approached the novel cue and did not approach the focal bat's trained cue. Focal bats therefore had the opportunity to learn to approach either the novel cue itself or the location from which the novel cue was broadcast. After post-tests, bats were given cue/location tests to determine whether bats learned to approach the novel cue or the side of the arena from which the novel cue had been broadcast. Cue/location tests consisted of two preference tests and two recognition tests with the speaker locations opposite those in the post-tests. Owing to a logistical problem, one bat in the UT-RA treatment and two bats in the UT-RS treatment did not receive cue/location tests. Within each treatment, we compared the proportion of trials bats preferentially approached the novel cue between the post-tests and the cue/location tests using paired t-tests. We then determined whether the preference for the cue differed significantly between playback locations. For the recognition tests, we compared the number of bats in each treatment that approached the novel cue in the opposite location using a Fisher's exact test. We also examined if bats differed in their recognition of the novel cue depending on whether they had recognized the novel cue in the post-tests using a Kruskal–Wallis one-way analysis of variance.
3. Results
(a). Pre-tests
Focal bats always approached the trained cue and did not approach the novel cue in any of the pre-tests.
(b). Experimental exposure trials
A few focal bats in each treatment approached the novel cue during the experimental exposure trials. More bats in the UT-RS treatment approached the novel cue than in the other treatments, but there was no statistically significant difference (Fisher's exact test: p = 0.095). Bats in the UT-RS treatment also approached the novel cue for more of the 100 experimental exposure trials (25.8 ± 10.1 trials) than bats in the RT-RS (2.2 ± 1.1 trials) or UT-RA treatments (11.0 ± 8.5 trials), but the response was not significantly predicted by treatment (negative binomial GLM: χ2 = 4.74, d.f. = 2, p = 0.093; figure 3).
Figure 3.
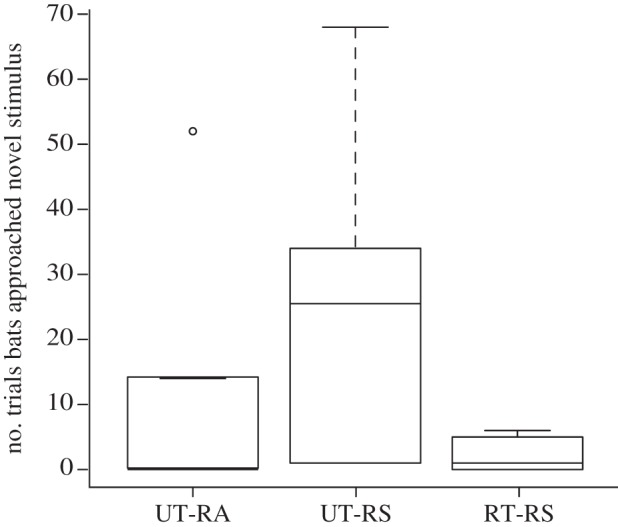
Boxplot of the number of experimental exposure trials (out of 100) for which focal bats in each treatment approached the novel cue over the trained cue.
(c). Post-tests
We found a significant effect of treatment on preference for the novel cue (GEE: Wald χ2 = 319, d.f. = 2, p < 0.001; figure 4a). When the novel cue had been demonstrated by a tutor bat, focal individuals whose trained cue was unreliable (UT-RS) approached the novel cue significantly more than individuals whose trained cue was reliable (RT-RS) (Wald = 8.73, p = 0.0031). When the trained cue was unreliable, bats that had a tutor (UT-RS) were significantly more likely to prefer the novel cue than bats that had no tutor (UT-RA) (Wald = 3829, p < 0.001). There was no significant difference between the RT-RS and UT-RA treatments (Wald = 0, p = 1.0). Therefore, reliability of the trained cue and the presence of a tutor in combination affected preference for the novel cue.
Figure 4.
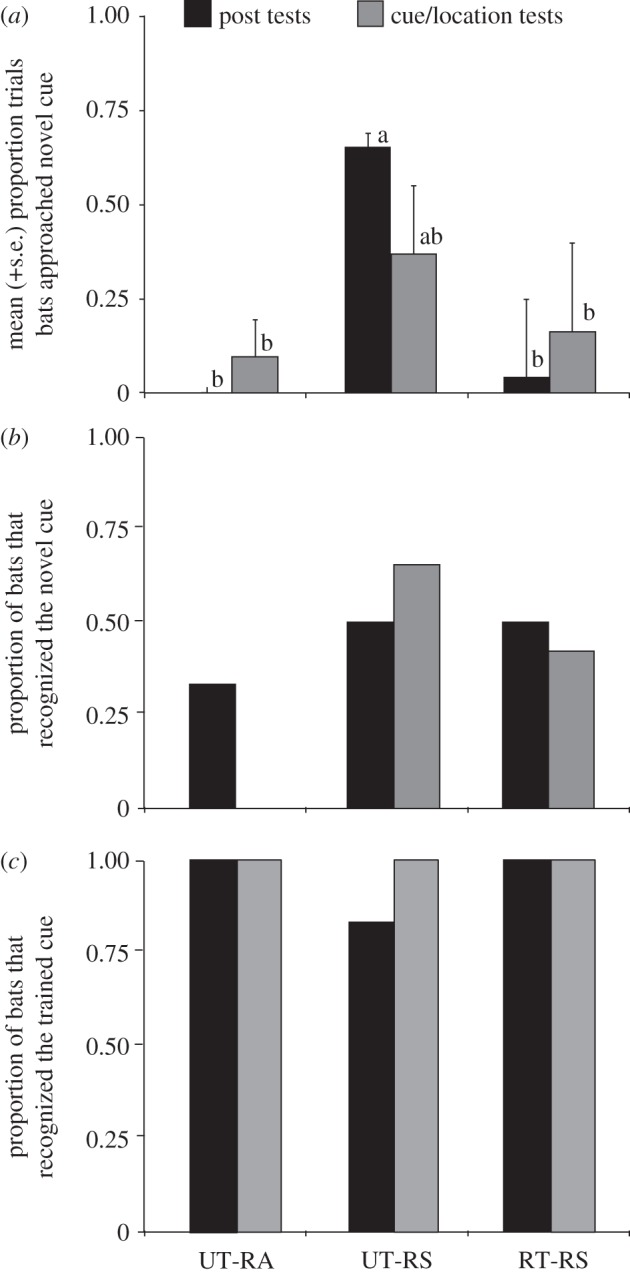
Post-test (black bars) and cue/location test (grey bars) results for the UT-RA, UT-RS and RT-RS treatments. (a) The mean and standard error of the proportion of trials for which focal bats approached the novel cue in two-speaker preference tests. Different letters indicate significant differences between groups. (b) The proportion of focal bats that approached the novel cue in single-speaker recognition tests. (c) The proportion of focal bats that approached the trained cue in single-speaker recognition tests.
We found no significant differences between treatments in recognition of the novel cue (Fisher's exact test: p = 1.0; figure 4b). A few bats in all of the treatments recognized the novel cue in the post-tests. Most of these bats had approached the novel cue during the experimental exposure trials. Neither the absence of a tutor nor a reliable trained cue deterred some bats from investigating the novel cue. There was also no significant difference between treatments in recognition of the trained cue (Fisher's exact test: p = 1.0; figure 4c). Bats that shifted their preference to the novel cue therefore maintained recognition of the trained cue.
(d). Cue/location tests
To determine whether bats had learned the cue or the location from which the cue was broadcast, we reversed the playback locations for the cue/location tests. We found no significant difference in preference for the novel cue between locations (paired t-tests; UT-RA t = −1, d.f. = 4, p = 0.37; RT-RS t = −0.69, d.f. = 5, p = 0.52; UT-RS t = 1.57, d.f. = 3, p = 0.23; figure 4a), indicating preference for the cue itself rather than the playback location.
In the cue/location recognition trials, the only bats to approach the novel cue in its new location were those that had a tutor present in the experimental exposure trials, a treatment difference that approached statistical significance (Fisher's exact test: p = 0.07; figure 4b). Bats that recognized the novel cue in the post-tests approached the novel cue when it was broadcast from a different location significantly more often than bats that had not recognized the novel cue in post-tests (Kruskal–Wallis: χ2 = 8.2, d.f. = 1, p = 0.004). All bats approached the trained cue when it was broadcast from the new location (figure 4c), further demonstrating bat response to the acoustic cue rather than the playback location.
4. Discussion
We found that bats whose self-acquired prey cues were unreliably associated with rewards were significantly more likely to approach a novel cue demonstrated by a conspecific tutor than either bats with reliable trained cues and a tutor, or bats with unreliable prey cues and no tutor. The reliability of self-acquired prey cues therefore affects bats' use of social information to learn novel prey cues. This result is consistent with multiple theoretical social learning strategies: ‘copy when uncertain’, ‘copy if better’, ‘copy when dissatisfied’ and ‘copy when asocial learning is costly’ [9,12]. The 50% reward schedule of the self-acquired prey cue could be interpreted as generating uncertainty about prey quality or environmental stability. Previous studies that have examined ‘copy when uncertain’, however, use uncertainty to refer to when the animal has very little, or conflicting, self-acquired information [31–35]. The bats in our experiment had substantial experience with their trained cue, and no conflicting information. We therefore feel that ‘copy when uncertain’, as it has been previously applied, is not likely to be the strategy exhibited by bats in this experiment.
‘Copy if better’ [12,36,37] is another relevant social learning strategy because the novel prey cue was always 100% rewarded, and thus ‘better’ than the 50% rewarded self-acquired prey cues in the unreliable treatments. We did not manipulate the reward schedule of the novel prey cue, and therefore did not directly test whether this is the strategy employed by the bats. ‘Copy if better’ is a relatively sophisticated social learning strategy, because it requires animals to evaluate and compare their own success with the demonstrator's success. A much simpler strategy is that individuals copy the behaviour of conspecifics when they are dissatisfied or when there are costs to individual learning [12,34,38]. ‘Copy when dissatisfied’ and ‘copy when asocial learning is costly’ are consistent with our experiment and do not require animals to assess the demonstrator's success. In T. cirrhosus, as in many animals, responding to a prey cue does not reliably result in a meal [39,40], making approaching prey cues costly and potentially resulting in dissatisfaction. ‘Copy when asocial learning is costly’ and ‘copy when dissatisfied’ are likely to be applicable in other taxa that use social information to learn novel foraging behaviours.
A few bats in all of our treatments approached the novel cue in the recognition tests. The availability of reliable known prey and the absence of a tutor do not appear to preclude investigation of novel prey. This disposition towards exploration of acoustic stimuli even in the absence of a conspecific tutor has been demonstrated previously for T. cirrhosus [22,26]. Only bats that had been exposed to a conspecific tutor, however, preferentially approached the novel cue over the trained cue when the tutor was removed. The presence of a tutor therefore appears to facilitate or reinforce a general tendency towards exploration of novel acoustic cues.
We found considerable individual variation in bat responses to novel prey cues and use of social information. For example, not all bats learned to approach novel cues, and of those that did, most learned the cue regardless of location, but a few were affected by playback location. All of the bats in this experiment were wild-captured adults and probably varied in their previous social and foraging experiences. Trachops cirrhosus generally roost together in small groups [41], and it is not unusual to capture two adult males in the same net in close succession (P. Jones 2011, personal observation), indicating the potential for transfer of foraging information in the wild. The variation we observed in this experiment may result in part from the previous social and foraging experiences of individuals in the wild. One of the advantages of conducting learning experiments with wild-caught adult animals is that it is more likely to encompass behaviourally relevant variation owing to previous experience. Results indicating social learning are therefore more robust and ecologically relevant, while at the same time revealing variation that is present in a wild population.
There is a growing literature on the factors that affect the use of social information when animals are presented with conflicting private (self-acquired) and public (social) information (e.g. [33,42–45]). Many animals, however, may not encounter such conflicts, but rather use social information to expand their behavioural repertoires. The study of when to use social information to learn novel prey or novel behaviours is crucial for understanding how novel behaviours can spread through populations and thereby create the potential for animal culture.
Acknowledgements
We would like to thank the staff of the Smithsonian Tropical Research Institute for their help with logistics and permits. Thanks to Christina Buelow, Jay Falk, Sarah Richman, Teague O'Mara, and Teia Schweizer and for help capturing and caring for bats. Stuart Dennis and Teague O'Mara provided statistical advice. John Ratcliffe and Kevin Laland provided helpful comments on the manuscript.
Capture in Soberanía National Park was approved by the Panamanian authorities (Autoridad Nacional del Ambiente, ANAM permit nos. SE/A-91-09; SE/A-95-10; SE/A-6-11; SE/A-46-11; SE/A-94-11). Animal care was conducted according to approved Institute for Animal Care and Use Committee protocols from the University of Texas (protocol AUP-2009-00138), and the Smithsonian Tropical Research Institute (protocol 20100816-1012-16).
Funding statement
This research was funded by an NSF GRFP (P.L.J.), NSF DDIG-1210655 (P.L.J.), and grants from the Smithsonian Tropical Research Institute (P.L.J.), American Society of Mammalogists (P.L.J.) and Animal Behavior Society (P.L.J.).
References
- 1.Fisher J, Hinde RA. 1949. The opening of milk bottles by birds. Br. Birds 42, 347–357 [Google Scholar]
- 2.Galef BG, Giraldeau LA. 2001. Social influences on foraging in vertebrates: causal mechanisms and adaptive functions. Anim. Behav. 61, 3–15 (doi:10.1006/anbe.2000.1557) [DOI] [PubMed] [Google Scholar]
- 3.Whiten A, Horner V, de Waal FBM. 2005. Conformity to cultural norms of tool use in chimpanzees. Nature 437, 737–740 (doi:10.1038/nature04047) [DOI] [PubMed] [Google Scholar]
- 4.Dugatkin LA. 1992. Sexual selection and imitation: females copy the mate choice of others. Am. Nat. 139, 1384–1389 (doi:10.1086/285392) [Google Scholar]
- 5.Dugatkin LA, Godin JGJ. 1992. Reversal of female mate choice by copying in the guppy (Poecilia reticulata). Proc. R. Soc. Lond. B 249, 179–184 (doi:10.1098/rspb.1992.0101) [DOI] [PubMed] [Google Scholar]
- 6.Schlupp I, Marler CA, Ryan MJ. 1994. Benefit to male sailfin mollies of mating with heterospecific females. Science 263, 373–374 (doi:10.1126/science.8278809) [DOI] [PubMed] [Google Scholar]
- 7.Galef BG, White DJ. 1998. Mate-choice copying in Japanese quail, Coturnix japonica. Anim. Behav. 55, 545–552 (doi:10.1006/anbe.1997.0616) [DOI] [PubMed] [Google Scholar]
- 8.Giraldeau LA, Valone TJ, Templeton JJ. 2002. Potential disadvantages of using socially acquired information. Phil. Trans. R. Soc. Lond. B 357, 1559–1566 (doi:10.1098/rstb.2002.1065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laland KN. 2004. Social learning strategies. Learn. Behav. 32, 4–14 (doi:10.3758/BF03196002) [DOI] [PubMed] [Google Scholar]
- 10.Boyd R, Richerson PJ. 1985. Culture and the evolutionary process. Chicago, IL: University of Chicago Press [Google Scholar]
- 11.Rogers A. 1988. Does biology constrain culture? Am. Anthropol. 90, 819–831 (doi:10.1525/aa.1988.90.4.02a00030) [Google Scholar]
- 12.Kendal RL, Coolen I, Laland KN. 2009. Adaptive trade-offs in the use of social and personal information. In Cognitive ecology II (eds Dukas R, Ratcliffe JM.), pp. 249–271 Chicago, IL: University of Chicago Press [Google Scholar]
- 13.Rendell L, Fogarty L, Hoppitt WJE, Morgan TJH, Webster MM, Laland KN. 2011. Cognitive culture: theoretical and empirical insights into social learning strategies. Trends Cogn. Sci. 15, 68–76 (doi:10.1016/j.tics.2010.12.002) [DOI] [PubMed] [Google Scholar]
- 14.Laland KN, Plotkin HC. 1991. Excretory deposits surrounding food sites facilitate social learning of food preferences in Norway rats. Anim. Behav. 41, 997–1005 (doi:10.1016/S0003-3472(05)80638-4) [Google Scholar]
- 15.Sherwin CM, Heyes CM, Nicola CJ. 2002. Social learning influences the preferences of domestic hens for novel food. Anim. Behav. 63, 933–942 (doi:10.1006/anbe.2002.2000) [Google Scholar]
- 16.Thornton A. 2008. Social learning about novel foods in young meerkats. Anim. Behav. 76, 1411–1421 (doi:10.1016/j.anbehav.2008.07.007) [Google Scholar]
- 17.Galef BG, Stein M. 1985. Demonstrator influence on observer diet preference: analyses of critical social interactions and olfactory signals. Anim. Learn. Behav. 13, 31–38 (doi:10.3758/BF03213362) [Google Scholar]
- 18.Galef BG. 2009. Strategies for social learning: testing predictions from formal theory. Adv. Stud. Behav. 39, 117–151 (doi:10.1016/S0065-3454(09)39004-X) [Google Scholar]
- 19.Wilkinson GS. 1992. Information transfer at evening bat colonies. Anim. Behav. 44, 501–518 (doi:10.1016/0003-3472(92)90059-I) [Google Scholar]
- 20.Gaudet CL, Fenton MB. 1984. Observational learning in three species of insectivorous bats (Chiroptera). Anim. Behav. 32, 385–388 (doi:10.1016/S0003-3472(84)80273-0) [Google Scholar]
- 21.Ratcliffe JM, ter Hofstede HM. 2005. Roosts as information centres: social learning of food preferences in bats. Biol. Lett. 1, 72–74 (doi:10.1098/rsbl.2004.0252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page RA, Ryan MJ. 2006. Social transmission of novel foraging behavior in bats: frog calls and their referents. Curr. Biol. 16, 1201–1205 (doi:10.1016/j.cub.2006.04.038) [DOI] [PubMed] [Google Scholar]
- 23.Wright GS, Wilkinson GS, Moss CF. 2011. Social learning of a novel foraging task by big brown bats, Eptesicus fuscus. Anim. Behav. 82, 1075–1083 (doi:10.1016/j.anbehav.2011.07.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuttle MD, Ryan MJ. 1981. Bat predation and the evolution of frog vocalizations in the Neotropics. Science 214, 677–678 (doi:10.1126/science.214.4521.677) [DOI] [PubMed] [Google Scholar]
- 25.Tuttle MD, Ryan MJ, Belwood JJ. 1985. Acoustical resource partitioning by two species of phyllostomid bats (Trachops cirrhosus and Tonatia sylvicola). Anim. Behav. 33, 1369–1370 (doi:10.1016/S0003-3472(85)80204-9) [Google Scholar]
- 26.Page RA, Ryan MJ. 2005. Flexibility in assessment of prey cue: frog-eating bats and frog calls. Proc. R. Soc. B 272, 841–847 (doi:10.1098/rspb.2004.2998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryan MJ, Tuttle MD. 1983. The ability of the frog-eating bat to discriminate among novel and potentially poisonous frog species using acoustic cues. Anim. Behav. 31, 827–833 (doi:10.1016/S0003-3472(83)80239-5) [Google Scholar]
- 28.R Core Team 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (http://www.R-project.org/) [Google Scholar]
- 29.Venables WN, Ripley BD. 2002. Modern applied statistics with S, 4th edn New York, NY: Springer [Google Scholar]
- 30.Højsgaard S, Halekoh U, Yan J. 2006. The R Package geepack for generalized estimating equations. J. Stat. Softw. 15, 1–11 [Google Scholar]
- 31.Boyd R, Richerson PJ. 1988. An evolutionary model of social learning: the effects of spatial and temporal variation. In Social learning: psychological and biological perspectives (eds Zentell TR, Galef BG.), pp. 29–48 Hillsdale, NJ: Lawrence Erlbaum [Google Scholar]
- 32.Visalberghi E, Fragaszy D. 1995. The behaviour of capuchin monkeys, Cebus apella, with novel food: the role of social context. Anim. Behav. 49, 1089–1095 (doi:10.1006/anbe.1995.0137) [Google Scholar]
- 33.van Bergen Y, Coolen I, Laland KN. 2004. Nine-spined sticklebacks exploit the most reliable source when public and private information conflict. Proc. R. Soc. Lond. B 271, 957–962 (doi:10.1098/rspb.2004.2684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galef BG, Jr, Dudley KE, Whiskin EE. 2008. Social learning of food preferences in ‘dissatisfied’ and ‘uncertain’ Norway rats. Anim. Behav. 75, 631–637 (doi:10.1016/j.anbehav.2007.06.024) [Google Scholar]
- 35.Grüter C, Czaczkes TJ, Ratnieks FLW. 2011. Decision making in ant foragers (Lasius niger) facing conflicting private and social information. Behav. Ecol. Sociobiol. 65, 141–148 (doi:10.1007/s00265-010-1020-2) [Google Scholar]
- 36.Schlag K. 1998. Why imitate, and if so, how? J. Econ. Theory 78, 130–156 (doi:10.1006/jeth.1997.2347) [Google Scholar]
- 37.Pike TW, Kendal JR, Rendell LE, Laland KN. 2010. Learning by proportional observation in a species of fish. Behav. Ecol. 21, 570–575 (doi:10.1093/beheco/arq025) [Google Scholar]
- 38.Grüter C, Ratnieks FLW. 2011. Honeybee foragers increase the use of waggle dance information when private information becomes unrewarding. Anim. Behav. 81, 949–954 (doi:10.1016/j.anbehav.2011.01.014) [Google Scholar]
- 39.Tuttle MD, Taft LK, Ryan MJ. 1982. Evasive behaviour of a frog in response to bat predation. Anim. Behav. 30, 393–397 (doi:10.1016/S0003-3472(82)80050-X) [Google Scholar]
- 40.Page RA, Ryan MJ. 2008. The effect of signal complexity on localization performance in bats that localize frog calls. Anim. Behav. 76, 761–769 (doi:10.1016/j.anbehav.2008.05.006) [Google Scholar]
- 41.Kalko EKV, Friemel D, Handley CO, Schnitzler HU. 1999. Roosting and foraging behavior of two Neotropical gleaning bats, Tonatia silvicola and Trachops cirrhosus (Phyllostomidae). Biotropica 31, 344–353 (doi:10.1111/j.1744-7429.1999.tb00146.x) [Google Scholar]
- 42.Duffy GA, Pike TW, Laland KN. 2009. Size-dependent directed social learning in nine-spined sticklebacks. Anim. Behav. 78, 371–375 (doi:10.1016/j.anbehav.2009.05.015) [Google Scholar]
- 43.Kendal JR, Rendell L, Pike TW, Laland KN. 2009. Nine-spined sticklebacks deploy a hill-climbing social learning strategy. Behav. Ecol. 20, 238–244 (doi:10.1093/beheco/arp016) [Google Scholar]
- 44.Pike TW, Laland KN. 2010. Conformist learning in nine-spined sticklebacks’ foraging decisions. Biol. Lett. 6, 466–468 (doi:10.1098/rsbl.2009.1014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Webster MM, Laland KN. 2011. Reproductive state affects reliance on public information in sticklebacks. Proc. R. Soc. B 278, 619–627 (doi:10.1098/rspb.2010.1562) [DOI] [PMC free article] [PubMed] [Google Scholar]


