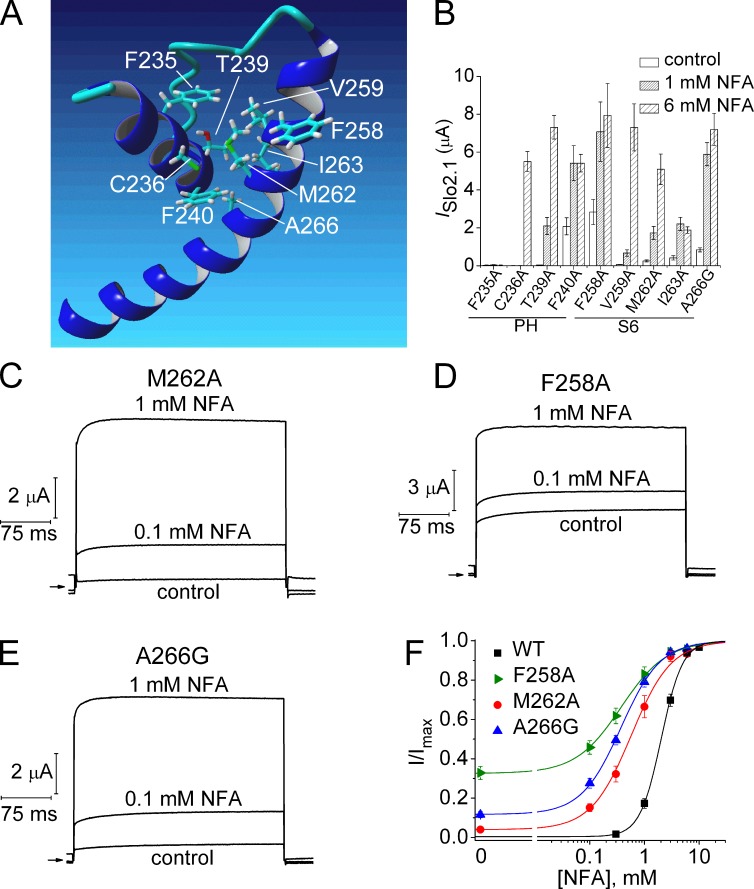
Figure 9.
Point mutation of S6 residues that face the pore helix exhibit enhanced sensitivity to NFA. (A) Homology model of pore helix and S6 segment of one Slo2.1 subunit highlighting potential residue interactions. (B) Peak outward currents measured at 0 mV in the absence (control) and presence of 1 and 6 mM NFA for channels with point mutations of the native residues in the pore helix (PH) or S6 highlighted in A (n = 5–7). (C–E) ISlo2.1 recorded from oocytes expressing M262A, F258A, and A266G Slo2.1 channels before (control) and after treatment with 0.1 and 1 mM NFA. Arrows indicate 0 current level. (F) [NFA]–response relationships for WT and gain-of-function S6 mutant channels. Data were fitted with a logistic equation (smooth curve). For WT (n = 9), EC50 = 2.1 ± 0.1 mM, nH = 2.4 ± 0.06; for F258A (n = 7): EC50 = 0.4 ± 0.05 mM, nH = 1.2 ± 0.08; for M262A (n = 6), EC50 = 0.66 ± 0.13 mM, nH = 1.4 ± 0.06; for A266G (n = 5), EC50 = 0.38 ± 0.03 mM, nH = 1.2 ± 0.03. Error bars indicate mean ± SEM.