Abstract
Vertebrate immune systems contain T cells bearing either alpha beta or gamma delta T-cell antigen receptors (TCRs). alpha beta T cells perform all well-characterized T-cell effector functions, while the biological functions of gamma delta + cells remain unclear. Of particular interest is the role of gamma delta + cells during epithelial infections, since gamma delta + cells are commonly abundant within epithelia. Eimeria spp. are intracellular protozoa that infect epithelia of most vertebrates, causing coccidiosis. This study shows that in response to Eimeria vermiformis, mice lacking alpha beta T cells display defects in protective immunity, while mice lacking gamma delta + cells display exaggerated intestinal damage, apparently due to a failure to regulate the consequences of the alpha beta T cell response. An immuno-downregulatory role during infection, and during autoimmune disease, may be a general one for gamma delta + cells.
Full text
PDF
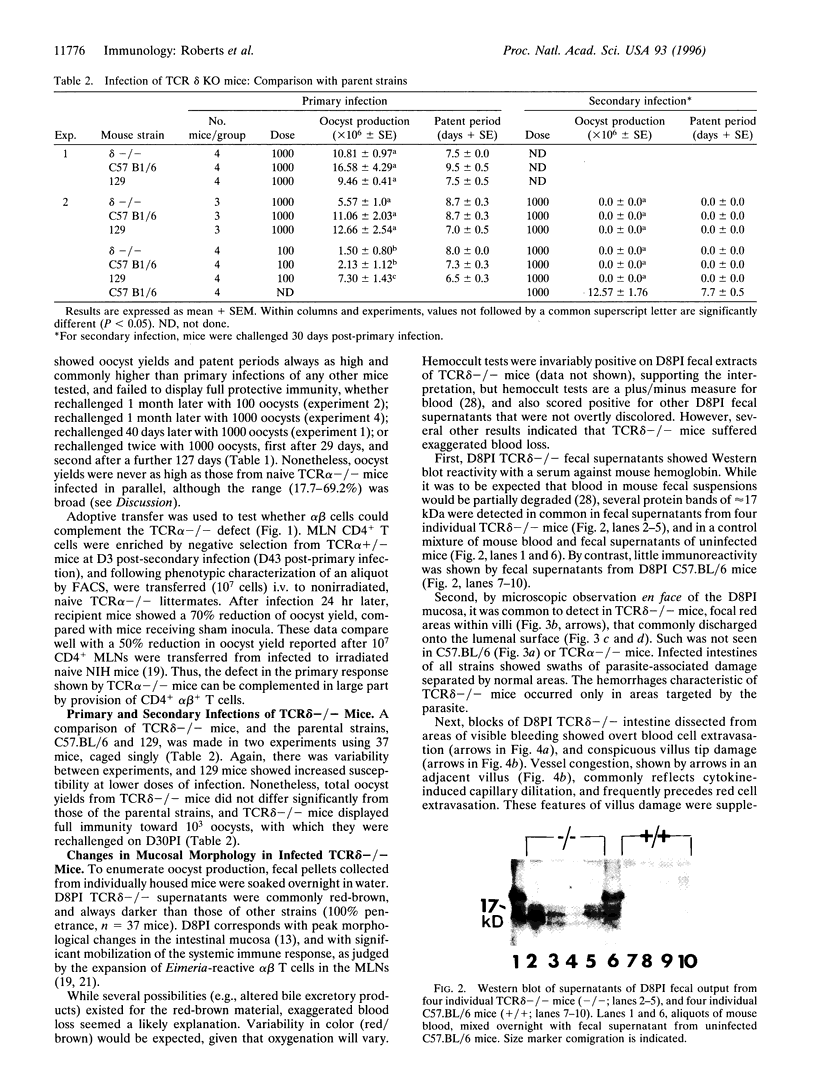
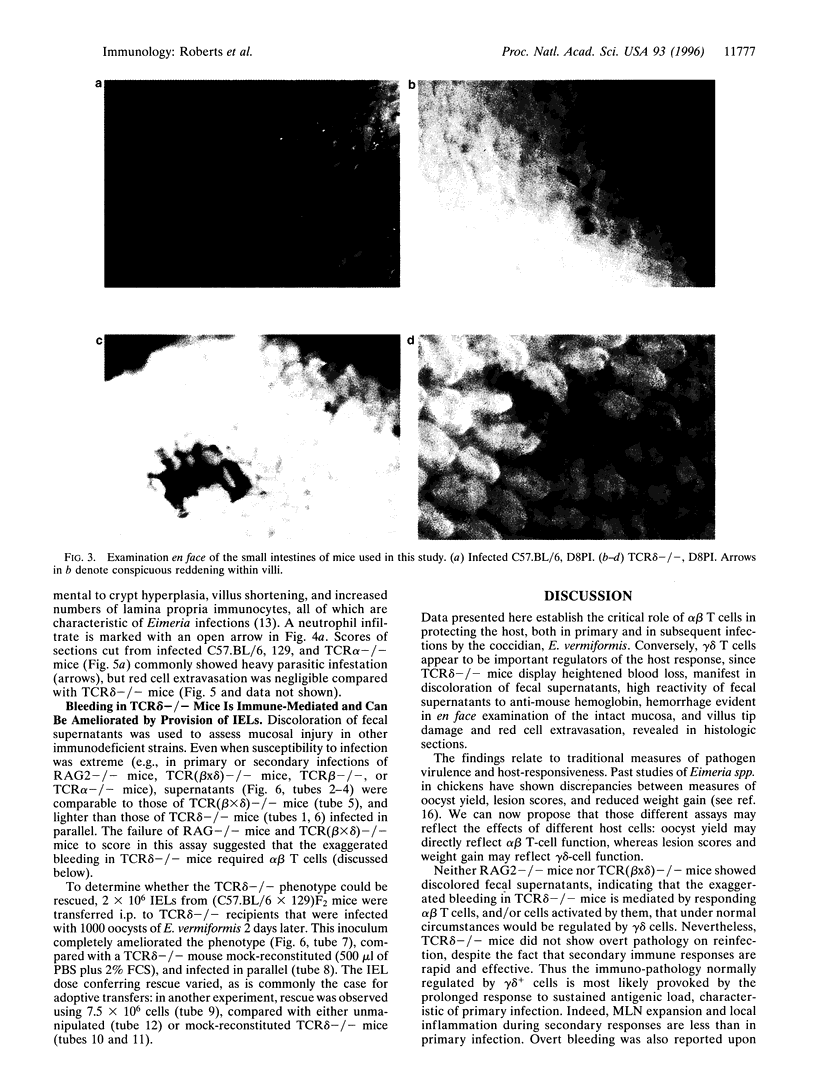
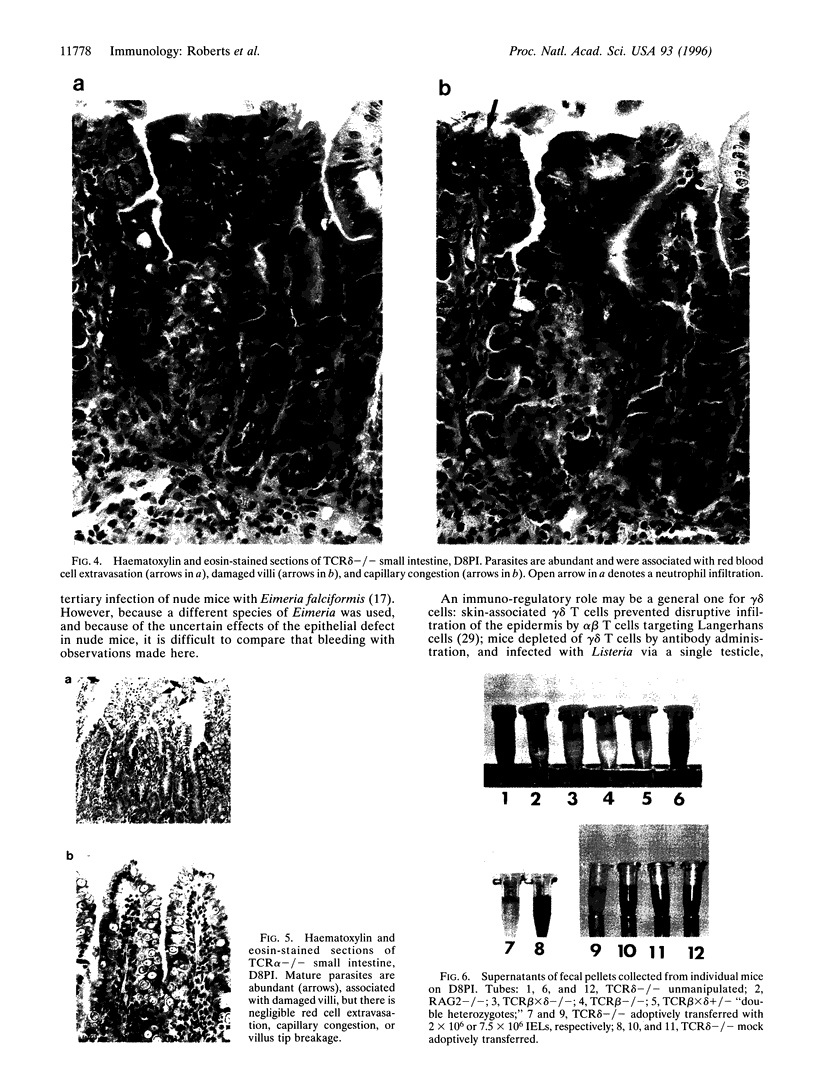

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blagburn B. L., Todd K. S., Jr Pathological changes and immunity associated with experimental Eimeria vermiformis infections in Mus musculus. J Protozool. 1984 Nov;31(4):556–561. doi: 10.1111/j.1550-7408.1984.tb05502.x. [DOI] [PubMed] [Google Scholar]
- Boismenu R., Havran W. L. Modulation of epithelial cell growth by intraepithelial gamma delta T cells. Science. 1994 Nov 18;266(5188):1253–1255. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- Bonneville M., Janeway C. A., Jr, Ito K., Haser W., Ishida I., Nakanishi N., Tonegawa S. Intestinal intraepithelial lymphocytes are a distinct set of gamma delta T cells. Nature. 1988 Dec 1;336(6198):479–481. doi: 10.1038/336479a0. [DOI] [PubMed] [Google Scholar]
- Bucy R. P., Chen C. L., Cihak J., Lösch U., Cooper M. D. Avian T cells expressing gamma delta receptors localize in the splenic sinusoids and the intestinal epithelium. J Immunol. 1988 Oct 1;141(7):2200–2205. [PubMed] [Google Scholar]
- Cepek K. L., Shaw S. K., Parker C. M., Russell G. J., Morrow J. S., Rimm D. L., Brenner M. B. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 1994 Nov 10;372(6502):190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Bègue B., Gagnon J., Meo T. The human intraepithelial lymphocyte marker HML-1 is an integrin consisting of a beta 7 subunit associated with a distinctive alpha chain. Eur J Immunol. 1992 Jan;22(1):273–277. doi: 10.1002/eji.1830220140. [DOI] [PubMed] [Google Scholar]
- Chien Y. H., Jores R., Crowley M. P. Recognition by gamma/delta T cells. Annu Rev Immunol. 1996;14:511–532. doi: 10.1146/annurev.immunol.14.1.511. [DOI] [PubMed] [Google Scholar]
- Deusch K., Lüling F., Reich K., Classen M., Wagner H., Pfeffer K. A major fraction of human intraepithelial lymphocytes simultaneously expresses the gamma/delta T cell receptor, the CD8 accessory molecule and preferentially uses the V delta 1 gene segment. Eur J Immunol. 1991 Apr;21(4):1053–1059. doi: 10.1002/eji.1830210429. [DOI] [PubMed] [Google Scholar]
- Eisenberg S. P., Evans R. J., Arend W. P., Verderber E., Brewer M. T., Hannum C. H., Thompson R. C. Primary structure and functional expression from complementary DNA of a human interleukin-1 receptor antagonist. Nature. 1990 Jan 25;343(6256):341–346. doi: 10.1038/343341a0. [DOI] [PubMed] [Google Scholar]
- Elson C. O., Sartor R. B., Tennyson G. S., Riddell R. H. Experimental models of inflammatory bowel disease. Gastroenterology. 1995 Oct;109(4):1344–1367. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- Findly R. C., Roberts S. J., Hayday A. C. Dynamic response of murine gut intraepithelial T cells after infection by the coccidian parasite Eimeria. Eur J Immunol. 1993 Oct;23(10):2557–2564. doi: 10.1002/eji.1830231027. [DOI] [PubMed] [Google Scholar]
- Fu Y. X., Roark C. E., Kelly K., Drevets D., Campbell P., O'Brien R., Born W. Immune protection and control of inflammatory tissue necrosis by gamma delta T cells. J Immunol. 1994 Oct 1;153(7):3101–3115. [PubMed] [Google Scholar]
- Goodman T., Lefrançois L. Expression of the gamma-delta T-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature. 1988 Jun 30;333(6176):855–858. doi: 10.1038/333855a0. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D., Griscelli C., Vassalli P. The gut-associated lymphoid system: nature and properties of the large dividing cells. Eur J Immunol. 1974 Jun;4(6):435–443. doi: 10.1002/eji.1830040610. [DOI] [PubMed] [Google Scholar]
- Itohara S., Mombaerts P., Lafaille J., Iacomini J., Nelson A., Clarke A. R., Hooper M. L., Farr A., Tonegawa S. T cell receptor delta gene mutant mice: independent generation of alpha beta T cells and programmed rearrangements of gamma delta TCR genes. Cell. 1993 Feb 12;72(3):337–348. doi: 10.1016/0092-8674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Jones B., Hayday A. Specificity and function of T cells bearing gamma delta receptors. Immunol Today. 1988 Mar;9(3):73–76. doi: 10.1016/0167-5699(88)91267-4. [DOI] [PubMed] [Google Scholar]
- Kilshaw P. J., Baker K. C. A unique surface antigen on intraepithelial lymphocytes in the mouse. Immunol Lett. 1988 Jun;18(2):149–154. doi: 10.1016/0165-2478(88)90056-9. [DOI] [PubMed] [Google Scholar]
- Klesius P. H., Hinds S. E. Strain-dependent differences in murine susceptibility to coccidia. Infect Immun. 1979 Dec;26(3):1111–1115. doi: 10.1128/iai.26.3.1111-1115.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komano H., Fujiura Y., Kawaguchi M., Matsumoto S., Hashimoto Y., Obana S., Mombaerts P., Tonegawa S., Yamamoto H., Itohara S. Homeostatic regulation of intestinal epithelia by intraepithelial gamma delta T cells. Proc Natl Acad Sci U S A. 1995 Jun 20;92(13):6147–6151. doi: 10.1073/pnas.92.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyes S., Carew E., Carding S. R., Janeway C. A., Jr, Hayday A. Diversity in T-cell receptor gamma gene usage in intestinal epithelium. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5527–5531. doi: 10.1073/pnas.86.14.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladel C. H., Hess J., Daugelat S., Mombaerts P., Tonegawa S., Kaufmann S. H. Contribution of alpha/beta and gamma/delta T lymphocytes to immunity against Mycobacterium bovis bacillus Calmette Guérin: studies with T cell receptor-deficient mutant mice. Eur J Immunol. 1995 Mar;25(3):838–846. doi: 10.1002/eji.1830250331. [DOI] [PubMed] [Google Scholar]
- Lefrançois L., Barrett T. A., Havran W. L., Puddington L. Developmental expression of the alpha IEL beta 7 integrin on T cell receptor gamma delta and T cell receptor alpha beta T cells. Eur J Immunol. 1994 Mar;24(3):635–640. doi: 10.1002/eji.1830240322. [DOI] [PubMed] [Google Scholar]
- Mahrt J. L., Shi Y. F. Murine major histocompatibility complex and immune response to Eimeria falciformis. Infect Immun. 1988 Jan;56(1):270–271. doi: 10.1128/iai.56.1.270-271.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M. N. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity ('celiac sprue'). Gastroenterology. 1992 Jan;102(1):330–354. [PubMed] [Google Scholar]
- Mesfin G. M., Bellamy J. E. Thymic dependence of immunity to Eimeria falciformis var. pragensis in mice. Infect Immun. 1979 Feb;23(2):460–464. doi: 10.1128/iai.23.2.460-464.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P., Arnoldi J., Russ F., Tonegawa S., Kaufmann S. H. Different roles of alpha beta and gamma delta T cells in immunity against an intracellular bacterial pathogen. Nature. 1993 Sep 2;365(6441):53–56. doi: 10.1038/365053a0. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Mizoguchi E., Grusby M. J., Glimcher L. H., Bhan A. K., Tonegawa S. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993 Oct 22;75(2):274–282. doi: 10.1016/0092-8674(93)80069-q. [DOI] [PubMed] [Google Scholar]
- Mukasa A., Hiromatsu K., Matsuzaki G., O'Brien R., Born W., Nomoto K. Bacterial infection of the testis leading to autoaggressive immunity triggers apparently opposed responses of alpha beta and gamma delta T cells. J Immunol. 1995 Aug 15;155(4):2047–2056. [PubMed] [Google Scholar]
- Parker C. M., Cepek K. L., Russell G. J., Shaw S. K., Posnett D. N., Schwarting R., Brenner M. B. A family of beta 7 integrins on human mucosal lymphocytes. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1924–1928. doi: 10.1073/pnas.89.5.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott K. L., Viney J. L., Kay G., Rastan S., Gardiner E. M., Chae S., Hayday A. C., Owen M. J. Lymphoid development in mice congenitally lacking T cell receptor alpha beta-expressing cells. Science. 1992 Jun 5;256(5062):1448–1452. doi: 10.1126/science.1604321. [DOI] [PubMed] [Google Scholar]
- Rose M. E., Hesketh P. Immunity to coccidiosis: T-lymphocyte- or B-lymphocyte-deficient animals. Infect Immun. 1979 Nov;26(2):630–637. doi: 10.1128/iai.26.2.630-637.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. E., Joysey H. S., Hesketh P., Grencis R. K., Wakelin D. Mediation of immunity to Eimeria vermiformis in mice by L3T4+ T cells. Infect Immun. 1988 Jul;56(7):1760–1765. doi: 10.1128/iai.56.7.1760-1765.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. E., Owen D. G., Hesketh P. Susceptibility to coccidiosis: effect of strain of mouse on reproduction of Eimeria vermiformis. Parasitology. 1984 Feb;88(Pt 1):45–54. doi: 10.1017/s0031182000054330. [DOI] [PubMed] [Google Scholar]
- Rose M. E., Wakelin D., Joysey H. S., Hesketh P. Immunity to coccidiosis: adoptive transfer in NIH mice challenged with Eimeria vermiformis. Parasite Immunol. 1988 Jan;10(1):59–69. doi: 10.1111/j.1365-3024.1988.tb00203.x. [DOI] [PubMed] [Google Scholar]
- Shinkai Y., Rathbun G., Lam K. P., Oltz E. M., Stewart V., Mendelsohn M., Charron J., Datta M., Young F., Stall A. M. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992 Mar 6;68(5):855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Shiohara T., Moriya N., Hayakawa J., Itohara S., Ishikawa H. Resistance to cutaneous graft-vs.-host disease is not induced in T cell receptor delta gene-mutant mice. J Exp Med. 1996 Apr 1;183(4):1483–1489. doi: 10.1084/jem.183.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockdale P. G., Stockdale M. J., Rickard M. D., Mitchell G. F. Mouse strain variation and effects of oocyst dose in infection of mice with Eimeria falciformis, a coccidian parasite of the large intestine. Int J Parasitol. 1985 Aug;15(4):447–452. doi: 10.1016/0020-7519(85)90032-3. [DOI] [PubMed] [Google Scholar]
- Todd K. S., Jr, Lepp D. L. The life cycle of Eimeria vermiformis Ernst, Chobotar and Hammond, 1971 in the mouse Mus musculus. J Protozool. 1971 May;18(2):332–337. doi: 10.1111/j.1550-7408.1971.tb03327.x. [DOI] [PubMed] [Google Scholar]
- Trejdosiewicz L. K., Smart C. J., Oakes D. J., Howdle P. D., Malizia G., Campana D., Boylston A. W. Expression of T-cell receptors TcR1 (gamma/delta) and TcR2 (alpha/beta) in the human intestinal mucosa. Immunology. 1989 Sep;68(1):7–12. [PMC free article] [PubMed] [Google Scholar]
- Viney J. L., Dianda L., Roberts S. J., Wen L., Mallick C. A., Hayday A. C., Owen M. J. Lymphocyte proliferation in mice congenitally deficient in T-cell receptor alpha beta + cells. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):11948–11952. doi: 10.1073/pnas.91.25.11948. [DOI] [PMC free article] [PubMed] [Google Scholar]