Abstract
Taenia solium is a zoonotic parasite that causes cysticercosis. The parasite is a major cause of human disease in impoverished communities where it is transmitted to humans from pigs which act as intermediate hosts. Vaccination of pigs to prevent transmission of T. solium to humans is an approach that has been investigated to control the disease. A recombinant vaccine antigen, TSOL18, has been remarkably successful at reducing infection of pigs with T. solium in several experimental challenge trials. The vaccine has been shown to eliminate transmission of naturally acquired T. solium in a field trial conducted in Africa. We recently reported that the vaccine was also effective in a field trial conducted in Peru. The TSOL18 recombinant antigen for each of these trials has been produced by expression in Escherichia coli. Here we discuss research that has been undertaken on the TSOL18 antigen and related antigens with a focus on improved methods of preparation of recombinant TSOL18 and optimized expression in Escherichia coli.
Keywords: cysticercosis, parasite, antigen, vaccine, Taenia solium, recombinant, pigs
Taenia solium is a zoonotic parasite that infects pigs and humans. It is prevalent in many developing countries of South America, Africa and Asia,1-5 causing a disease known as cysticercosis. Vaccination of pigs has been investigated as a measure to control transmission of T. solium to humans.6,7 Recently, we have demonstrated that a recombinant vaccine, produced in Escherichia coli, is highly effective at reducing T. solium pig infections under field conditions.8
A number of host protective, recombinant antigens have been investigated as potential vaccines for use in pigs, including the S3P vaccine9 and also antigens cloned from T. solium oncospheres. Larval oncosphere antigens have provided the highest levels of protection. Three different protective antigens have been cloned from T. solium oncospheres and these are designated TSOL18,10 TSOL45–1A11 and TSOL16.12 Each of these antigens has been shown to induce near complete protection in vaccinated pigs challenged with T. solium under experimental conditions.13-15 The TSOL18 antigen has shown the greatest potential. TSOL18 has been assessed in five separate pig vaccine trials, under experimental conditions conducted by four independent groups of scientists in four different countries. In the challenge trials, TSOL18 consistently provided vaccinated pigs with nearly complete protection against T. solium infection, achieving 100% and 99.5% protection in Mexico,13 99.9% in Peru,15 100% in Cameroon13 and 99.3% in Honduras.16 In addition, the TSOL18 antigen was shown to completely eliminate transmission of T. solium to pigs in a vaccine field trial conducted in the Mayo-Danay district of north Cameroon,17 a region previously identified as having a high prevalence of T. solium.18,19 A second, more recent vaccine field trial was undertaken in rural villages in the Morropon province of north-east Peru,8 and again vaccination greatly reduced the total number of parasite cysts in immunized pigs (99.7% reduction compared with control pigs).
The TSOL18 antigen used in the field trial described by Jayashi et al.,8 and in the other laboratory and field-based vaccine trials, has been produced by expression in Escherichia coli. The antigen was expressed as a fusion protein with glutathione S-transferase (GST) via a pGEX plasmid vector.20 The GST fusion partner allows purification of TSOL18 from lysed E. coli by affinity chromatography on glutathione sepharose. Prior to the antigen being tested in vaccine trials, expression of the TSOL18 antigen in E. coli culture required optimization. Initially the cDNA sequence corresponding to the full open reading frame of the associated tsol18 gene was expressed at only very low levels and the TSOL18‑GST protein could not be obtained at a high level of purity.21 Subsequently, the TSOL18 cDNA was truncated, deleting the N‑terminal nucleotides encoding a predicted secretory signal of 18 amino acids.21 These amino acids contained a high proportion of hydrophobic residues. Removal of these amino acids resulted in substantial increases in the expression of soluble TSOL18-GST by E. coli.
We have applied this genetic construct design strategy (removal of short stretches of hydrophobic amino acid residues at the N and C termini of parasite proteins) to improve the expression of related cestode antigens in E. coli.14,21,22 This strategy was adopted after it was discovered, serendipitously, that a similar approach improved the stability of a related parasite antigen expressed in E. coli. The 45W protein from Taenia ovis23 was among the first vaccine antigens found to be expressed at higher levels in E. coli following deletion of cDNA sequence encoding a carboxy terminal region comprising mainly hydrophobic amino acids.24 Following characterization of the 45W gene encoding the cloned mRNA,25 it became apparent that the original 45W construct expressing the T. ovis vaccine antigen also lacked another hydrophobic region at the N-terminus, comprising 16 amino acids. The absence of this latter region was likely to have resulted from incomplete reverse transcription during cDNA library construction. In effect, the genetically modified construct (45WB/X) was truncated at both the amino and carboxy ends,24 both of which contained a high proportion of hydrophobic residues. The carboxy terminal truncation of the cDNA had been prepared somewhat fortuitously by restriction digestion and plasmid cloning. These modifications were prepared prior to the development of PCR methods; with the advent of PCR it has been possible to truncate recombinant antigens by precise removal of codon regions corresponding to specific amino acids.14,21,22 Recent advances in gene synthesis technology26 now makes it possible to reduce the effort required to prepare modified genetic constructs and, where gene sequence is available, there would be no requirement for isolation of mRNA encoding the antigen of interest.
The improved expression of the cestode antigens in E. coli observed using the truncated gene strategy is consistent with the observations of others that have identified the occurrence of hydrophobic patches on a protein as one of the main features that hinders expression of recombinant proteins in E. coli.27,28 Hydrophobic amino acid stretches have been shown to significantly affect the ability to clone, express and purify recombinant proteins.29
Investigations have been undertaken seeking to identify defined protective epitope(s) of TSOL18. Antibodies raised by TSOL18 in vaccinated pigs were shown to be able to kill the T. solium parasite in in vitro culture and it appears that the major protective immune mechanism induced by vaccination with TSOL18 is antibody and complement-mediated killing of infecting parasites.30 We have investigated whether linear or conformational determinants are the principal antibody specificities raised by TSOL18 in vaccinated pigs. Truncated, recombinant forms of TSOL18 (essentially two halves of the antigen, Fig. 1) were unable to inhibit binding of anti-TSOL18 antibodies to TSOL18 from vaccinated pigs known to be protected against T. solium infection.31 From these studies it was concluded that the dominant antibody specificities, and probably host protective specificities, of TSOL18 are directed toward conformational epitopes. These findings indicated that since the host protective epitopes of TSOL18 appear to be conformational, options for the development of a vaccine based on defined epitopes produced as synthetic peptides are limited. However, the TSOL18 antigen is relatively small (112 amino acids) and it may soon be possible that the whole antigen could be synthesized at a purity and cost that would allow it to be used directly as a vaccine.
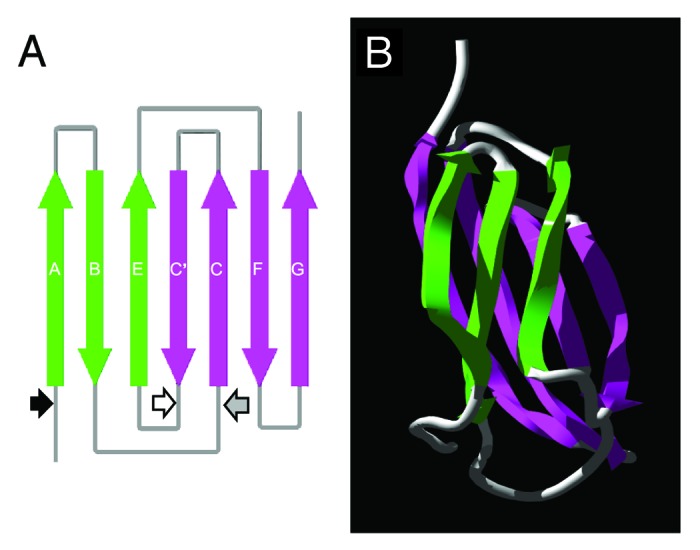
Figure 1. Diagrammatic representation of the predicted fibronectin type III (FnIII) domain within the TSOL18 antigen. (A) Schematic diagram of the secondary structure and position of β strands in the FnIII domain. Large arrows denote the direction (N to C terminus) of β strands within the antiparallel β sheets (green, pink) of the FnIII domain. Small arrows represent the amino acid positions of each genetic truncation of the TSOL18 recombinant antigen: black, TSOL18 (amino acids 19–130) lacking secretory signal; white, N-terminal portion of TSOL18 (amino acids 19–89); gray, carboxy terminal portion of TSOL18 (amino acids 65–130). The two genetic truncations of TSOL18 represented by the white and gray arrows (amino and carboxy portions) are likely to have disrupted the protective, conformational epitopes of TSOL18, since either alone or when combined, they were incapable of inhibiting any detectable reactivity of anti-TSOL18 immune serum to TSOL18, indicating that the host protective epitopes of TSOL18 are conformational.31 (B) Tertiary structure of the FnIII domain. Protein structure predictions were performed using Phyre39 and Swiss-Pdb Viewer.40 Reproduced (in part) with permission from Kyngdon et al.41
The TSOL18 recombinant antigen that is used as a vaccine comprises of a single, predicted fibronectin type III (FnIII) domain (Fig. 1). An FnIII domain has also been described in all other protective oncosphere antigens that have been identified from other cestode parasites.32 The FnIII domain is found in multi-domain proteins from other eukaryotic organisms including cell adhesion molecules, extracellular matrix proteins and cell surface receptors.33 Fibronectins and tenascins contain multiple tandem repeats of the FnIII domain and are associated with cell adhesion, migration and proliferation as well as wound healing, tumorigenesis and embryonic development.34 The three-dimensional structure of the tenth FnIII module of fibronectin has been determined and contains seven β-strands forming a sandwich of two anti-parallel β-sheets.35
It has been noted that single domains of proteins are more likely to be successfully expressed in E. coli than large, multi-domain proteins.36 This observation is consistent with genetic construct design strategies applied to TSOL18 and the other cestode antigens, which resulted in successful expression of the antigens in E. coli, most likely since they are relatively simple molecules consisting of one or two FnIII domains and a secretory signal, with some also having a C-terminal transmembrane domain. On this basis it has been possible to modify the genetic constructs to delete these regions of the antigens and retain the conformational epitopes within the FnIII domain which confer host protective immunity (Fig. 1).
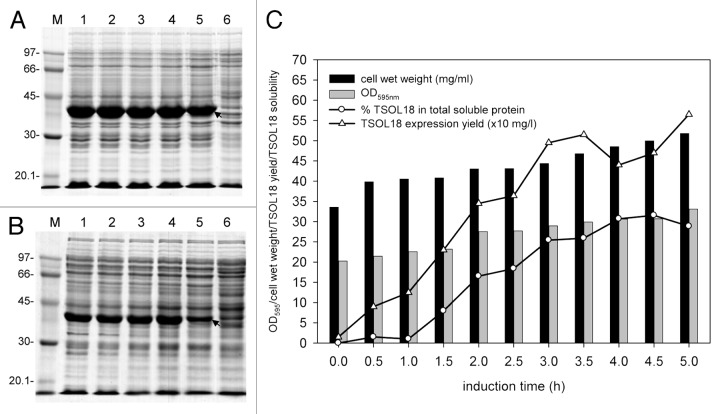
Experimental investigations have shown that the use of E. coli for TSOL18 antigen production may be a feasible approach for future large scale production of the vaccine. However, under standard culture conditions, TSOL18 is often produced as insoluble inclusion bodies in E. coli,21 as is also the case for related cestode antigens.37,38 To investigate this problem, culture conditions for E. coli expressing truncated TSOL18 fused to GST were initially optimized in shake flasks in order to identify the best conditions in which the antigen was produced (Fig. 2). Simple adjustment of E. coli culture conditions, by reducing the temperature of induction of protein expression from 37°C (Fig. 2A and B, lane 5) to 30°C (Fig. 2A and B, lanes 1–4), resulted in an increase in the proportion of soluble TSOL18 produced. Increased amounts of soluble TSOL18 in extracts of total soluble proteins from lysed E. coli allow increased recoveries of the antigen by affinity chromatography. Optimization of E. coli culture has led to improved expression of TSOL18 and establishment of reproducible culture conditions in a fermentor, providing consistent yields of the antigen (Fig. 2C). These investigations have demonstrated that high cell densities, together with TSOL18 expression, can be achieved using the pGEX/E. coli expression system in a fermentor. Consistent yields and improved solubility of TSOL18-GST were observed following optimization of fermentation conditions (Fig. 2C). We also determined that a balance between cell growth and recombinant protein synthesis is necessary to maintain high expression levels in fermentor culture. Various fermentor operating parameters may require fine tuning to maintain this balance, but use of the pGEX vector and E. coli to express TSOL18 in a fermentor provides a robust means of producing the antigen for large scale production.
Figure 2. Expression of TSOL18-GST in E. coli. A, B, comparison of E. coli cultures expressing TSOL18-GST that were incubated at different temperatures and induced using various concentrations of isopropyl β-D thiogalactopyranoside (IPTG). SDS-PAGE of insoluble proteins (A) and soluble proteins (B) from bacterial lysates. 1–4, samples from cultures incubated at 30°C and induced with 0.2 mM, 0.5 mM, 1 mM and 2 mM IPTG respectively. 5, sample from culture induced with 0.2 mM IPTG and incubated at 37°C. 6, no IPTG. M, protein markers (kDa). Arrows denote position of TSOL18-GST. C, analysis of samples from an optimized fermentor culture containing E. coli expressing TSOL18-GST. A 10 L Biostat B Plus fermentor (Sartorius) was used in the fermentation and expression was induced with 0.2 mM IPTG when the OD595nm reached 20 units (0 h in [C]). The culture was maintained at pH 7.5 and 50% dissolved oxygen (DO) concentration. The DO concentration was maintained using a cascade of agitation between 200–800 rpm and air supplied at 0.4–20 l/min via a Biostat Bplus micro-DCU. Pure oxygen was blended into the air at high cell densities and was regulated via the control unit. Temperature was set at 37°C and reduced to 30°C at the initiation of induction with IPTG. Super broth (35 g soy peptone, 20 g yeast extract, 5 g NaCl and 100 mg ampicillin per liter) was used in the fermentation. Super broth feed (containing 20% glycerol) was added at 5 ml/min when OD595nm reached 3 units. Half hourly samples were removed from the fermentation and used to determine OD595nm, cell wet weight, % TSOL18 in total soluble proteins from lysed E.coli and TSOL18 expression yields (C). The percentage of TSOL18-GST in total soluble E.coli proteins was determined by scanning densitometry (Molecular Dynamics) of culture samples separated by SDS-PAGE, following removal of medium by centrifugation, lysis by sonication in phosphate buffered saline and removal of insoluble solids by centrifugation at 10,000 g. TSOL18 expression yields were determined from the soluble protein fractions of culture samples by scanning densitometry and comparison to standards of known mass on SDS-PAGE.
The operational characteristics for production of the TSOL18 vaccine by bacterial fermentation have been defined. They have enabled preparation of the antigen for use in vaccine field trials and provide the basis upon which TSOL18 may be produced on a large-scale. The ultimate goal of these studies relates to enabling the widespread use of the TSOL18 antigen as a veterinary vaccine to reduce human infection with cysticercosis. Attention to the development of strategies for large scale production of the antigen may play an important role in achieving these aims.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia.
Submitted
09/23/12
Revised
11/23/12
Accepted
11/26/12
Footnotes
Previously published online: www.landesbioscience.com/journals/bioe/article/23003
References
- 1.García HH, Gonzalez AE, Evans CA, Gilman RH, Cysticercosis Working Group in Peru Taenia solium cysticercosis. Lancet. 2003;362:547–56. doi: 10.1016/S0140-6736(03)14117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mafojane NA, Appleton CC, Krecek RC, Michael LM, Willingham AL., 3rd The current status of neurocysticercosis in Eastern and Southern Africa. Acta Trop. 2003;87:25–33. doi: 10.1016/S0001-706X(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 3.Zoli A, Shey-Njila O, Assana E, Nguekam JP, Dorny P, Brandt J, et al. Regional status, epidemiology and impact of Taenia solium cysticercosis in Western and Central Africa. Acta Trop. 2003;87:35–42. doi: 10.1016/S0001-706X(03)00053-6. [DOI] [PubMed] [Google Scholar]
- 4.Ito A, Wandra T, Yamasaki H, Nakao M, Sako Y, Nakaya K, et al. Cysticercosis/taeniasis in Asia and the Pacific. Vector Borne Zoonotic Dis. 2004;4:95–107. doi: 10.1089/1530366041210756. [DOI] [PubMed] [Google Scholar]
- 5.Willingham AL, 3rd, Wu HW, Conlan J, Satrija F. Combating Taenia solium cysticercosis in Southeast Asia an opportunity for improving human health and livestock production. Adv Parasitol. 2010;72:235–66. doi: 10.1016/S0065-308X(10)72009-1. [DOI] [PubMed] [Google Scholar]
- 6.Lightowlers MW. Eradication of Taenia solium cysticercosis: a role for vaccination of pigs. Int J Parasitol. 2010;40:1183–92. doi: 10.1016/j.ijpara.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Lightowlers MW. Eradication of Taenia solium cysticercosis: a role for vaccination of pigs. Int J Parasitol. 1999;29:811–7. doi: 10.1016/S0020-7519(99)00051-X. [DOI] [PubMed] [Google Scholar]
- 8.Jayashi CM, Kyngdon CT, Gauci CG, Gonzalez AE, Lightowlers MW. Successful immunization of naturally reared pigs against porcine cysticercosis with a recombinant oncosphere antigen vaccine. Vet Parasitol. 2012;188:261–7. doi: 10.1016/j.vetpar.2012.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sciutto E, Fragoso G, de Aluja AS, Hernández M, Rosas G, Larralde C. Vaccines against cysticercosis. Curr Top Med Chem. 2008;8:415–23. doi: 10.2174/156802608783790839. [DOI] [PubMed] [Google Scholar]
- 10.Gauci CG, Flisser A, Lightowlers MW. A Taenia solium oncosphere protein homologous to host-protective Taenia ovis and Taenia saginata 18 kDa antigens. Int J Parasitol. 1998;28:757–60. doi: 10.1016/S0020-7519(98)00034-4. [DOI] [PubMed] [Google Scholar]
- 11.Gauci CG, Lightowlers MW. Alternative splicing and sequence diversity of transcripts from the oncosphere stage of Taenia solium with homology to the 45W antigen of Taenia ovis. Mol Biochem Parasitol. 2001;112:173–81. doi: 10.1016/S0166-6851(00)00364-9. [DOI] [PubMed] [Google Scholar]
- 12.Gauci C, Lightowlers MW. Molecular cloning of genes encoding oncosphere proteins reveals conservation of modular protein structure in cestode antigens. Mol Biochem Parasitol. 2003;127:193–8. doi: 10.1016/S0166-6851(03)00005-7. [DOI] [PubMed] [Google Scholar]
- 13.Flisser A, Gauci CG, Zoli A, Martinez-Ocaña J, Garza-Rodriguez A, Dominguez-Alpizar JL, et al. Induction of protection against porcine cysticercosis by vaccination with recombinant oncosphere antigens. Infect Immun. 2004;72:5292–7. doi: 10.1128/IAI.72.9.5292-5297.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gauci CG, Jayashi CM, Gonzalez AE, Lackenby J, Lightowlers MW. Protection of pigs against Taenia solium cysticercosis by immunization with novel recombinant antigens. Vaccine. 2012;30:3824–8. doi: 10.1016/j.vaccine.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez AE, Gauci CG, Barber D, Gilman RH, Tsang VC, Garcia HH, et al. Vaccination of pigs to control human neurocysticercosis. Am J Trop Med Hyg. 2005;72:837–9. [PubMed] [Google Scholar]
- 16.Lightowlers MW. Cestode vaccines: origins, current status and future prospects. Parasitology. 2006;133(Suppl):S27–42. doi: 10.1017/S003118200600179X. [DOI] [PubMed] [Google Scholar]
- 17.Assana E, Kyngdon CT, Gauci CG, Geerts S, Dorny P, De Deken R, et al. Elimination of Taenia solium transmission to pigs in a field trial of the TSOL18 vaccine in Cameroon. Int J Parasitol. 2010;40:515–9. doi: 10.1016/j.ijpara.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Assana E, Amadou F, Thys E, Lightowlers MW, Zoli AP, Dorny P, et al. Pig-farming systems and porcine cysticercosis in the north of Cameroon. J Helminthol. 2010;84:441–6. doi: 10.1017/S0022149X10000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Assana E, Zoli A, Sadou HA, Nguekam, Vondou L, Pouedet MSR, et al. Prevalence of porcine cysticercosis in Mayo-Danay (North Cameroon) and Mayo-Kebbi (Southwest Chad) Rev Elev Med Vet Pays Trop. 2001;54:123–7. [Google Scholar]
- 20.Smith DB, Johnson KS. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 21.Gauci C, Jenkins D, Lightowlers MW. Strategies for optimal expression of vaccine antigens from Taeniid cestode parasites in Escherichia coli. Mol Biotechnol. 2011;48:277–89. doi: 10.1007/s12033-010-9368-0. [DOI] [PubMed] [Google Scholar]
- 22.Gauci C, Vural G, Oncel T, Varcasia A, Damian V, Kyngdon CT, et al. Vaccination with recombinant oncosphere antigens reduces the susceptibility of sheep to infection with Taenia multiceps. Int J Parasitol. 2008;38:1041–50. doi: 10.1016/j.ijpara.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson KS, Harrison GB, Lightowlers MW, O’Hoy KL, Cougle WG, Dempster RP, et al. Vaccination against ovine cysticercosis using a defined recombinant antigen. Nature. 1989;338:585–7. doi: 10.1038/338585a0. [DOI] [PubMed] [Google Scholar]
- 24.Lightowlers MW, Waterkeyn JG, Rothel JS, Gauci CG, Harrison GB. Host-protective fragments and antibody binding epitopes of the Taenia ovis 45W recombinant antigen. Parasite Immunol. 1996;18:507–13. doi: 10.1046/j.1365-3024.1996.d01-20.x. [DOI] [PubMed] [Google Scholar]
- 25.Waterkeyn JG, Lightowlers MW, Coppel R, Cowman AF. Characterization of the gene family encoding a host-protective antigen of the tapeworm Taenia ovis. Mol Biochem Parasitol. 1995;73:123–31. doi: 10.1016/0166-6851(94)00104-U. [DOI] [PubMed] [Google Scholar]
- 26.Gustafsson C, Minshull J, Govindarajan S, Ness J, Villalobos A, Welch M. Engineering genes for predictable protein expression. Protein Expr Purif. 2012;83:37–46. doi: 10.1016/j.pep.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murby M, Samuelsson E, Nguyen TN, Mignard L, Power U, Binz H, et al. Hydrophobicity engineering to increase solubility and stability of a recombinant protein from respiratory syncytial virus. Eur J Biochem. 1995;230:38–44. doi: 10.1111/j.1432-1033.1995.tb20531.x. [DOI] [PubMed] [Google Scholar]
- 28.Yoder S, Cao C, Ugen KE, Dao ML. High-level expression of a truncated wall-associated protein A from the dental cariogenic Streptococcus mutans. DNA Cell Biol. 2000;19:401–8. doi: 10.1089/10445490050085898. [DOI] [PubMed] [Google Scholar]
- 29.Goh CS, Lan N, Douglas SM, Wu B, Echols N, Smith A, et al. Mining the structural genomics pipeline: identification of protein properties that affect high-throughput experimental analysis. J Mol Biol. 2004;336:115–30. doi: 10.1016/j.jmb.2003.11.053. [DOI] [PubMed] [Google Scholar]
- 30.Kyngdon CT, Gauci CG, Rolfe RA, Velásquez Guzmán JC, Farfán Salazar MJ, Verástegui Pimentel MR, et al. In vitro oncosphere-killing assays to determine immunity to the larvae of Taenia pisiformis, Taenia ovis, Taenia saginata, and Taenia solium. J Parasitol. 2006;92:273–81. doi: 10.1645/GE-619R.1. [DOI] [PubMed] [Google Scholar]
- 31.Assana E, Gauci CG, Kyngdon CT, Zoli AP, Dorny P, Geerts S, et al. Antibody responses to the host-protective Taenia solium oncosphere protein TSOL18 in pigs are directed against conformational epitopes. Parasite Immunol. 2010;32:399–405. doi: 10.1111/j.1365-3024.2009.01197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lightowlers MW, Flisser A, Gauci CG, Heath DD, Jensen O, Rolfe R. Vaccination against cysticercosis and hydatid disease. Parasitol Today. 2000;16:191–6. doi: 10.1016/S0169-4758(99)01633-6. [DOI] [PubMed] [Google Scholar]
- 33.Koide A, Bailey CW, Huang X, Koide S. The fibronectin type III domain as a scaffold for novel binding proteins. J Mol Biol. 1998;284:1141–51. doi: 10.1006/jmbi.1998.2238. [DOI] [PubMed] [Google Scholar]
- 34.Tucker RP, Chiquet-Ehrismann R. Evidence for the evolution of tenascin and fibronectin early in the chordate lineage. Int J Biochem Cell Biol. 2009;41:424–34. doi: 10.1016/j.biocel.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Main AL, Harvey TS, Baron M, Boyd J, Campbell ID. The three-dimensional structure of the tenth type III module of fibronectin: an insight into RGD-mediated interactions. Cell. 1992;71:671–8. doi: 10.1016/0092-8674(92)90600-H. [DOI] [PubMed] [Google Scholar]
- 36.Gräslund S, Sagemark J, Berglund H, Dahlgren LG, Flores A, Hammarström M, et al. The use of systematic N- and C-terminal deletions to promote production and structural studies of recombinant proteins. Protein Expr Purif. 2008;58:210–21. doi: 10.1016/j.pep.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Dempster RP, Robinson CM, Harrison GB. Parasite vaccine development: large-scale recovery of immunogenic Taenia ovis fusion protein GST-45W(B/X) from Escherichia coli inclusion bodies. Parasitol Res. 1996;82:291–6. doi: 10.1007/s004360050116. [DOI] [PubMed] [Google Scholar]
- 38.Manderson D, Dempster R, Chisti Y. A recombinant vaccine against hydatidosis: production of the antigen in Escherichia coli. J Ind Microbiol Biotechnol. 2006;33:173–82. doi: 10.1007/s10295-005-0046-3. [DOI] [PubMed] [Google Scholar]
- 39.Kelley LA, Sternberg MJ. Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc. 2009;4:363–71. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- 40.Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18:2714–23. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 41.Kyngdon CT, Gauci CG, Gonzalez AE, Flisser A, Zoli A, Read AJ, et al. Antibody responses and epitope specificities to the Taenia solium cysticercosis vaccines TSOL18 and TSOL45-1A. Parasite Immunol. 2006;28:191–9. doi: 10.1111/j.1365-3024.2006.00820.x. [DOI] [PubMed] [Google Scholar]