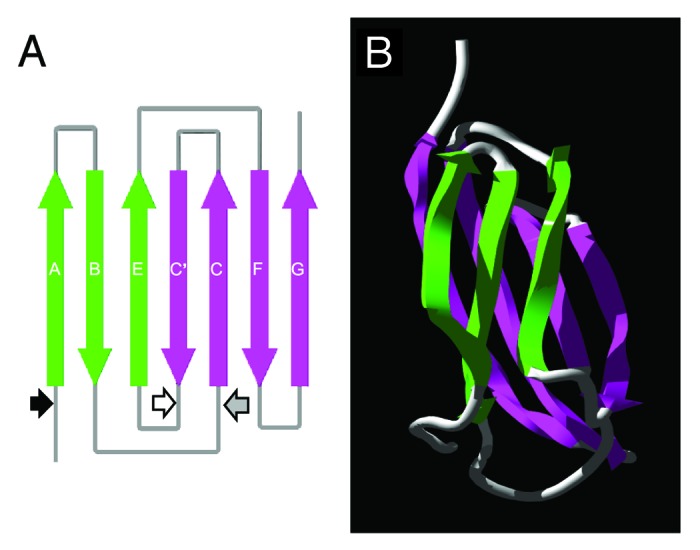
Figure 1. Diagrammatic representation of the predicted fibronectin type III (FnIII) domain within the TSOL18 antigen. (A) Schematic diagram of the secondary structure and position of β strands in the FnIII domain. Large arrows denote the direction (N to C terminus) of β strands within the antiparallel β sheets (green, pink) of the FnIII domain. Small arrows represent the amino acid positions of each genetic truncation of the TSOL18 recombinant antigen: black, TSOL18 (amino acids 19–130) lacking secretory signal; white, N-terminal portion of TSOL18 (amino acids 19–89); gray, carboxy terminal portion of TSOL18 (amino acids 65–130). The two genetic truncations of TSOL18 represented by the white and gray arrows (amino and carboxy portions) are likely to have disrupted the protective, conformational epitopes of TSOL18, since either alone or when combined, they were incapable of inhibiting any detectable reactivity of anti-TSOL18 immune serum to TSOL18, indicating that the host protective epitopes of TSOL18 are conformational.31 (B) Tertiary structure of the FnIII domain. Protein structure predictions were performed using Phyre39 and Swiss-Pdb Viewer.40 Reproduced (in part) with permission from Kyngdon et al.41
