Fig. 5. REGγregulates SirT1 autophagic function by inhibiting its binding to and deacetylating autophagy complex.
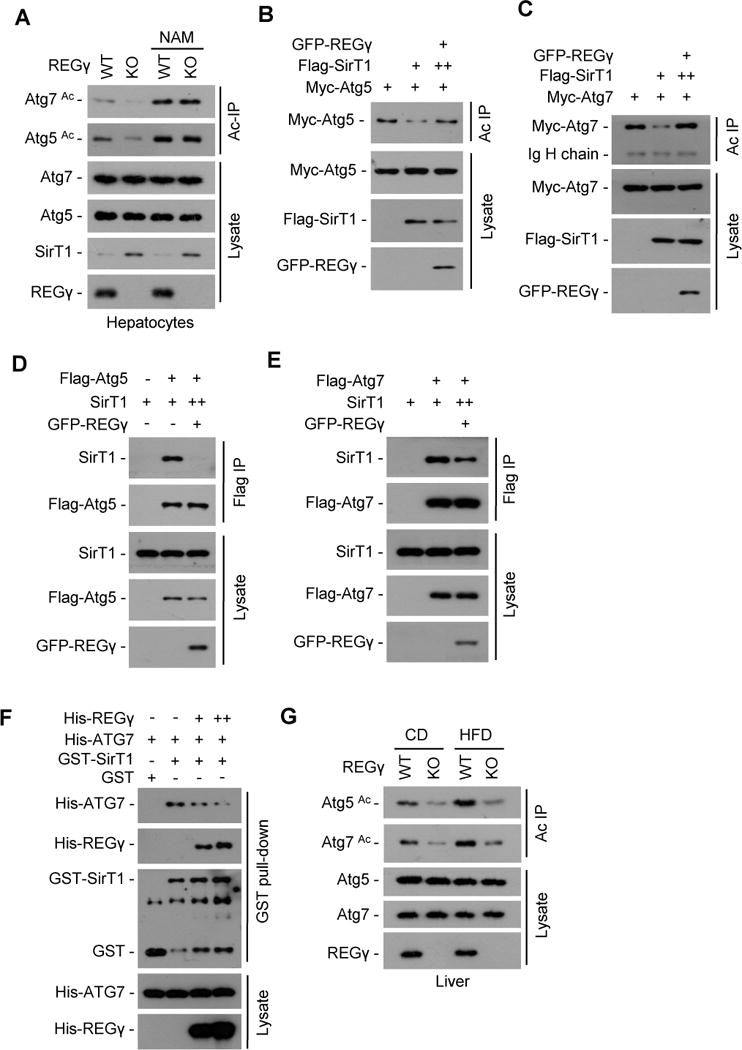
(A) REGγ regulates acetylation states of Atg5 and Atg7 through SirT1. REGγ-WT and -KO primary hepatocytes were treated with or without NAM (5 mM, 12 h), cell extracts were immunoprecipitated by anti-acetyl-lysine antibody (Ac IP). The immunoprecipitates were subjected to Western blotting with anti-Atg5 or -Atg7 antibody. (B–C) 293T cells were cotransfected with indicated plasmids and acetylated proteins were immunoprecipitated by anti-acetyl-lysine antibody (Ac IP). To ensure equal expression of SirT1, more SirT1 plasmid DNA (++) was cotransfected with REGγ. The acetylation levels of Atg5/7 protein were determined by Western blot using anti-Myc tag antibody. (D–E) REGγ inhibits SirT1 binding to Atg5/7. 293T cells transfected with indicated plasmids and Flag-tagged Atg5/7 were immunoprecipitated using FLAG-M2 agarose beads followed by Western blot. To ensure equal expression of SirT1, more SirT1 plasmid DNA (++) was cotransfected with REGγ. (F) REGγ inhibits SirT1-Atg7 binding in vitro. GST-SirT1 was incubated with His-Atg7 in the presence or absence of His-REGγ for 4 h at 4° C, followed by GST pulldown and Western blot. (G) REGγ regulates Atg5 and Atg7 acetylation in vivo. The acetylation status of Atg5 and Atg7 were measured in hepatocytes from REGγ-WT and -KO mice on the CD or HFD for 19 weeks. Liver tissue lysates were immunoprecipitated by anti-acetyl-lysine antibody (Ac IP), and the endogenous acetylation levels of Atg5 and Atg7 proteins were determined by Western blot using anti-Atg5 or -Atg7 antibodies. See also Fig. S5.
