Fig. 7. Energy starvation dissociates REGγ-SirT1, unleashing SirT1 to associate with Atg proteins for autophagy activation.
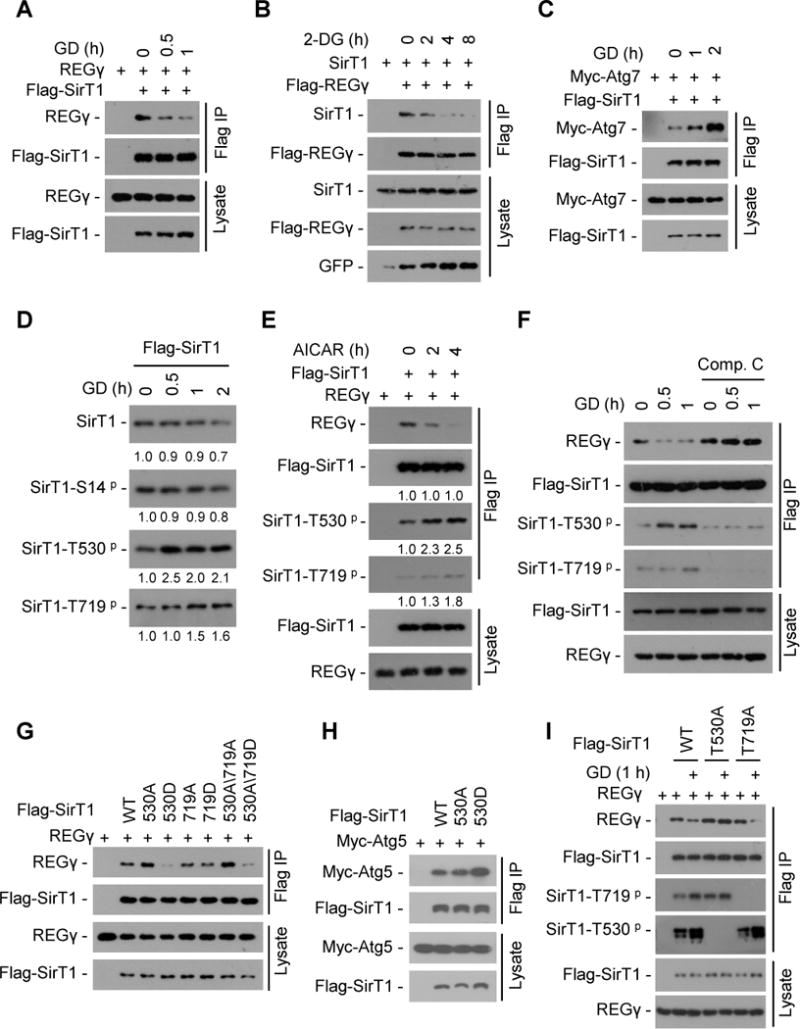
(A) 293T cells were transiently transfected with Flag-SirT1 and REGγ followed by glucose free medium (glucose deprivation, GD) treatment. SirT1 was immunoprecipitated using FLAG-M2 beads and co-precipitation of REGγ was detected by Western blot. (B) 293T cells were transfected with Flag-REGγ, SirT1 and GFP plasmids followed by 2-DG (12.5 mM) treatment. REGγ was immunoprecipitated using FLAG-M2 beads and co-precipitation of SirT1 was detected by Western blot. (C) 293T cells were transiently transfected with Flag-SirT1 and Myc-Atg7 followed by GD treatment. SirT1 was immunoprecipitated using FLAG-M2 beads and co-precipitated Atg7 was detected by Western blot with anti-Myc antibody. (D) Time course of SirT1 phosphorylation in response to glucose starvation. Flag-SirT1 transfected 293T cells were starved for GD for indicated times. SirT1 was immunoprecipitated with FLAG-M2 beads and phosphorylation of SirT1 were determined by Western blot with antibodies against SirT1 phosphorylation at S14, T530 or T719. Relative levels of phosphorylated and total SirT1 were quantified by densitometry of bands and are presented below the blots. (E) Activation of AMPK induces SirT1-phosphorylation coupled with REGγ-SirT1 dissociation. Flag-SirT1 and REGγ were transfected into 293T cells for 24 h, and then cells were treated with or without AMPK activator AICAR (200 μM). SirT1 was immunoprecipitated with FLAG-M2 beads and the immunoprecipitated complex were examined by Western blot. Relative protein levels were quantified by densitometry of bands and are presented below the blots. (F) AMPK inhibitor Compound C prevents glucose starvation-induced dissociation of REGγ-SirT1. Flag-SirT1 transfected 293T cells were treated with Compound C (10 μM) for 1 h followed by a 0.5–1 h GD treatment. SirT1 was immunoprecipitated by FLAG-M2 beads and co-precipitated REGγ were determined by Western blot. (G–H) Phosphorylation-mimetic mutation (T530D) in SirT1 regulates its association with REGγ and Atg5 proteins. Interaction of Flag-SirT1 mutants with REGγ (G) and Atg5 (H) were determined by cotransfection of indicated plasmids into 293T cells followed by immunoprecipitation using FLAG-M2 beads and Western blot. (I) SirT1 Thr530 phosphorylation is responsible for GD-induced REGγ-SirT1 dissociation. Flag-SirT1 mutants and REGγ cotransfected 293T cells were treated with or without GD for 1 h. SirT1 was immunoprecipitated using FLAG-M2 beads and the immunoprecipitated complex were examined by Western blotting. See also Fig. S7.
