Generation of an ER-targeted Cameleon reporter protein enables the analysis of Ca2+ accumulation and dynamics in the lumen of the ER in plant cells.
Abstract
In planta, very limited information is available about how the endoplasmic reticulum (ER) contributes to cellular Ca2+ dynamics and homeostasis. Here, we report the generation of an ER-targeted Cameleon reporter protein suitable for analysis of Ca2+ accumulation and dynamics in the lumen of the ER in plant cells. Using stably transformed Arabidopsis (Arabidopsis thaliana) plants expressing this reporter protein, we observed a transiently enhanced accumulation of Ca2+ in the ER in response to stimuli inducing cytosolic Ca2+ rises in root tip cells. In all experimental conditions, ER Ca2+ dynamics were substantially different from those monitored in the cytosol. A pharmacological approach enabled us to evaluate the contribution of the different ER-resident Ca2+-ATPase classes in the regulation of the ER Ca2+ homeostasis. Taken together, our results do not provide evidence for a role of the ER as a major source that releases Ca2+ for stimulus-induced increases in cytosolic Ca2+ concentration. Instead, our results show that the luminal ER Ca2+ elevations typically follow cytosolic ones, but with distinct dynamics. These findings suggest fundamental differences for the function of the ER in cellular Ca2+ homeostasis in plants and animals.
In plants, rises in cytosolic Ca2+ concentration ([Ca2+]cyt) occur in response to both biotic and abiotic stimuli (Hetherington and Brownlee, 2004; McAinsh and Pittman, 2009; Kudla et al.., 2010; Bose et al., 2011). Depending on the stimulus, these rises can display the form of a single transient or repetitive Ca2+ oscillations and are commonly designated as “Ca2+ signatures” (Webb et al., 1996; Allen et al., 2000, 2001; Sanders et al., 2002; Young et al., 2006; Kudla et al., 2010).
The generation and shaping of [Ca2+]cyt signatures depends on fine-tuning of Ca2+ influxes and effluxes occurring at both the plasma membrane (PM) and membranes of the different subcellular compartments (Pittman and Hirschi, 2003; Hetherington and Brownlee, 2004; Dodd et al., 2010; Spalding and Harper, 2011). The opening of Ca2+-permeable influx channels in response to a stimulus will release Ca2+ into the cytosol and cause the generation of a Ca2+ spike, while the activity of Ca2+ efflux transporters (H+-Ca2+ antiporters and Ca2+-ATPases) will return the [Ca2+]cyt to resting concentrations (McAinsh and Pittman, 2009; Bonza and De Michelis, 2011; Spalding and Harper, 2011).
Recently, the development and application of genetically encoded Ca2+ reporter proteins like Cameleons has allowed the study of Ca2+ dynamics in several compartments with organ, tissue, and single-cell resolution (Allen et al., 1999; Monshausen et al., 2008; Yang et al., 2008; Sieberer et al., 2009; Costa et al., 2010; Rincón-Zachary et al., 2010; Tanaka et al., 2010; Michard et al., 2011; Krebs et al., 2012; Loro et al., 2012; Behera et al., 2013). Cameleons are fluorescence resonance energy transfer (FRET)-based indicators in which two GFP variants, cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP; or circularly permuted variants of YFP), are linked together by the Ca2+-binding protein calmodulin (CaM) and a CaM-binding peptide (Miyawaki et al., 1997). Binding of Ca2+ to the Ca2+-responsive elements alters the efficiency of FRET, allowing for quantitative measurements of Ca2+ dynamics. Different Cameleon variants have been developed since the first report about this reporter protein (Miyawaki et al., 1997). This involved, for example, advanced versions with improved fluorescence, larger changes in FRET upon Ca2+ binding, and a broad range of Ca2+ affinities (Palmer and Tsien, 2006). Moreover, mutational modification of the original Cameleon in the case of the D family of indicators ensured that their function is no longer perturbed by large excesses of native CaM (Palmer et al., 2006). The use of Cameleons in plants has allowed the study of cytosolic (Krebs et al., 2012), nuclear (Sieberer et al., 2009; Krebs et al., 2012), peroxisomal (Costa et al., 2010, 2013), and mitochondrial (Loro et al., 2012, 2013) Ca2+ dynamics in specific plant organs and single cells. However, despite all these advances, very limited data have been reported regarding in vivo analyses of endoplasmic reticulum (ER) Ca2+ dynamics in plants (Iwano et al., 2009).
In animal cells, the ER represents an important Ca2+ storage organelle in which the free Ca2+ concentration varies between 50 and 500 μm (Coe and Michalak, 2009). Ca2+ release from the ER is involved in many different processes, including exocytosis, contraction, metabolism, regulation of transcription, fertilization, and apoptosis. The major Ca2+ entry pathway in electrically nonexcitable cells is represented by the “store-operated Ca2+ entry” (SOCE; Feske et al., 2012). Here, PM-localized calcium release-activated channels are activated in response to the emptying of intracellular ER Ca2+ stores (Parekh and Putney, 2005; Carrasco and Meyer, 2010). Moreover, in several mammalian cell types upon stimulation, Ca2+ is released from the ER into the cytosol through the activity of different classes of ER-resident Ca2+-permeable channels activated by second messengers such as inositol 1,4,5-trisphosphate (IP3) and cyclic adenosine diphosphoribose (Parekh and Putney, 2005; Berridge, 2009; Galione and Chuang, 2012). In sharp contrast, for plant cells, no precise data on ER Ca2+ concentration ([Ca2+]ER) are available (Stael et al., 2012), and in vivo investigations about the Ca2+ storage properties of the ER have remained very limited (Iwano et al., 2009).
Moreover, SOCE has not been reported in plants, and stromal interaction molecule (STIM) proteins, which are central components of SOCE, are not encoded in the genomes of higher plants (Collins and Meyer, 2011). Despite some biochemical evidence for Ca2+ release in response to IP3, nicotinic acid adenine dinucleotide phosphate, and cyclic adenosine diphosphoribose (Muir and Sanders, 1997; Navazio et al., 2000, 2001), electrophysiological analyses supporting the existence of voltage-gated ER-localized Ca2+ channels (Klüsener et al., 1995, 1997), homologous for the respective IP3 and ryanodine receptors, are missing in higher plants (Wheeler and Brownlee, 2008; Kudla et al., 2010). All considered, this situation suggests that the role of the ER for cellular Ca2+ dynamics may be fundamentally different in plants as compared with animal cells.
Plants contain two major types of Ca2+ pumps named ECA (for ER-type Ca2+-ATPase) and ACA (for autoinhibited Ca2+-ATPase; Geisler et al., 2000; Sze et al., 2000; Bonza and De Michelis, 2011). Plant genomes encode different isoforms of both ECAs and ACAs. These are often coexpressed in certain cell types, and consequently, different cellular membranes in the same cell contain multiple Ca2+-ATPase isoforms (Bonza and De Michelis, 2011). In Arabidopsis (Arabidopsis thaliana), immunological and membrane fractionation studies provided evidence for an ER localization of at least two distinct Ca2+ pumps, ECA1 and ACA2 (Liang et al., 1997; Harper et al., 1998; Liang and Sze, 1998; Hong et al., 1999; Hwang et al., 2000). Moreover, additional ECA and ACA isoforms are predicted to be ER localized (Geisler et al., 2000; Sze et al., 2000, Baxter et al., 2003; Bonza and De Michelis, 2011). Biochemical characterization revealed that ECA1 and ACA2, besides different regulatory properties, also have different affinities for Ca2+, with one-half saturation concentration for free Ca2+ in the submicromolar and micromolar range, respectively (Liang and Sze, 1998; Hwang et al., 2000; Sze et al., 2000; Wu et al., 2002; Bonza and De Michelis, 2011), suggesting their participation in different aspects of ER Ca2+ regulation. The main predicted function for the ER Ca2+-ATPases is Ca2+ loading into the ER lumen (Corbett and Michalak, 2000; Persson and Harper, 2006). However, how exactly these ER Ca2+-ATPases contribute to ER and cytosolic Ca2+ dynamics awaits further investigation.
Here, we report the successful development and application of a genetically encoded Cameleon Ca2+ reporter protein for the in vivo analyses of Ca2+ dynamics in the ER lumen of Arabidopsis by the combination of mammalian and plant targeting signals. Using this tool, we were able to monitor ER Ca2+ dynamics in root cells with organ, tissue, and cell resolution. This approach allowed us to evaluate in vivo the contribution of the different Ca2+-ATPase classes in [Ca2+]ER homeostasis. Taken together, our data do not support a role of the ER as a major Ca2+-releasing source for stimulus-induced increases in cytosolic Ca2+ concentration. Instead, our results show that the luminal ER Ca2+ transients typically follow cytosolic increases, but with distinct dynamics. These findings suggest fundamental differences for the function of the ER in cellular Ca2+ homeostasis in plants and animals.
RESULTS AND DISCUSSION
Generation of a Cameleon-Based Reporter Protein for Monitoring Ca2+ Dynamics in the ER of Plants
In order to enable analyses of Ca2+ dynamics in the ER of plant cells, we first took advantage of the available D1ER Cameleon Ca2+ reporter protein that had already been successfully used to study Ca2+ dynamics in the ER of mammalian cells (Palmer et al., 2004; Luciani et al., 2009; Jiménez-Moreno et al., 2010). In this reporter construct, fusion of the D1 reporter protein with a mammalian calreticulin signal sequence and a KDEL ER-retention signal results in specific localization of the Ca2+ reporter protein to the mammalian ER (Palmer et al., 2004). Although a modified version of D1ER (CRT-D1ER) was correctly localized at the ER in plant cells, we could detect neither an increase nor a decrease of [Ca2+]ER in response to specific stimuli (for details concerning construct production and localization analysis, see Supplemental Text S1 and Supplemental Fig. S1, respectively).
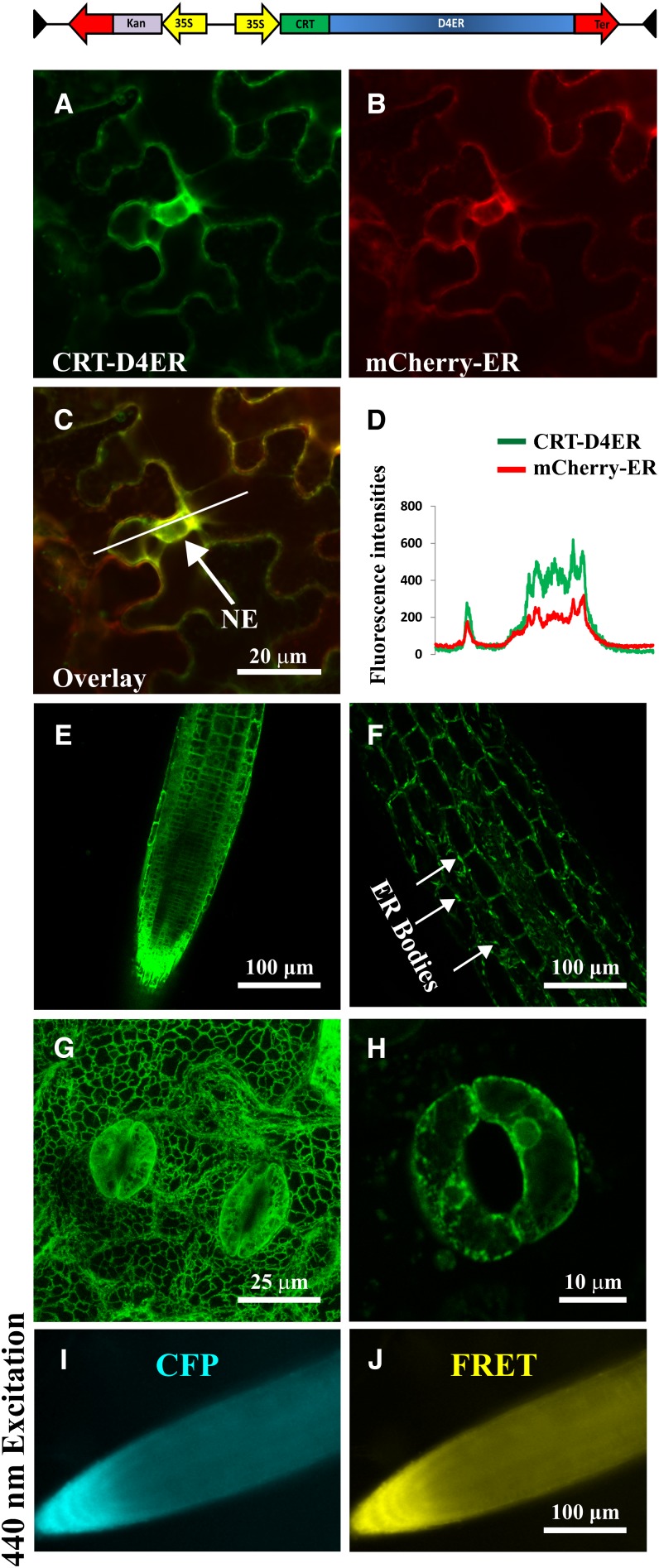
Since D1 Cameleon has a biphasic Ca2+ dependency in vitro (dissociation constant = 0.8 and 60 μm; Palmer and Tsien, 2006), we supposed that the resting [Ca2+]ER in the analyzed cells was above these values. Therefore, we decided to evaluate an alternative probe with a different affinity for Ca2+. The Cameleon D4 variant has an in vitro dissociation constant for Ca2+ of 195 μm (Palmer et al., 2006) and was very recently used by two groups to monitor free Ca2+ levels in the ER lumen of mammalian cells (Ravier et al., 2011; Kipanyula et al., 2012). To efficiently target the D4 probe to the lumen of plant cell ER, we followed the same cloning strategy adopted for the production of the CRT-D1ER construct. The Arabidopsis CALRETICULIN1A (Christensen et al., 2010) signal peptide was fused upstream of the D4ER Cameleon reporter (Kipanyula et al., 2012), and the entire coding sequence was placed under the control of a single cauliflower mosaic virus (CaMV) 35S promoter. The resulting construct, CRT-D4ER (Fig. 1), was transiently expressed in tobacco (Nicotiana benthamiana) leaf epidermal cells (Fig. 1, A–C). Comparison with the localization of nWAK2-mCherry-HDEL (for a full description of the construct, see Nelson et al., 2007) as an ER marker that was coexpressed in these cells revealed that nWAK2-mCherry-HDEL and CRT-D4ER fluorescences fully merged in the nuclear envelope of tobacco epidermal cells (Fig. 1, C and D). The CRT-D4ER reporter construct was subsequently transformed into Arabidopsis plants, and 15 independent stable transgenic lines were selected. Figure 1, E to H, provides representative images of CRT-D4ER Arabidopsis transgenic plants, demonstrating that the probe was expressed in root (Fig. 1E), hypocotyl (Fig. 1F), and mature leaf cells (Fig. 1G). In these different tissues/organs, the typical ER morphology was clearly recognized (Boevink et al., 1999; Brandizzi et al., 2002), with 1- to 5-μm ER fusiform bodies (Hawes et al., 2001; Matsushima et al., 2002, 2003; Nelson et al., 2007) detected in the hypocotyl cells (Fig. 1G). The CRT-D4ER probe was also abundantly expressed in guard cells (Fig. 1, G and H). In order to investigate the Ca2+ reporter protein conformation status in resting conditions, we imaged the root tip of transgenic CRT-D4ER seedlings by means of a wide-field fluorescence microscope (excited with a 436/20-nm light wavelength) for the simultaneous acquisition of the CFP and YFP emission wavelengths. In root tip cells expressing CRT-D4ER, the fluorescences of both CFP and the FRET acceptor (YFP in the D4) were detected and were clearly above the level of organ autofluorescence (Fig. 1, I and J). Similar results were obtained by confocal microscopic analysis performed both in leaves and root cells (data not shown).
Figure 1.
Subcellular distribution of CRT-D4ER in plant cells and simultaneous detection of CFP and FRET emissions. The top panel shows a schematic structure of the CRT-D4ER Cameleon probe. A to C, Confocal images of tobacco agroinfiltrated epidermal cells cotransformed with CRT-D4ER and the ER marker nWAK2-mCherry-HDEL (Nelson et al., 2007). A, Cameleon YFP fluorescence in tobacco epidermal cells. B, mCherry fluorescence in the same cells shown in A. C, The YFP signal of CRT-D4ER colocalizes with mCherry, as revealed by colocalization of the two fluorescence signals at the level of the nuclear envelope (NE). D, Plot profile of YFP and mCherry fluorescences corresponding to the marked white line shown in C. E to H, Confocal microscopy analyses revealed efficient CRT-D4ER YFP expression in different organs of Arabidopsis stable transgenic plants with a subcellular distribution showing typical ER features. E, Root tip cells. F, Hypocotyl cells, showing the presence of fusiform bodies. G, Three-dimensional maximum projection of a mature leaf region. H, Stomata guard cells. I and J, Representative root tip of a CRT-D4ER seedling excited with 440-nm light for FRET detection. I, CFP emission. J, FRET emission. The simultaneous detection of the two emissions demonstrates that CFP was not quenched and YFP was properly excited trough FRET.
CRT-D4ER Allows the Visualization of ER Ca2+ Dynamics in Root Cells
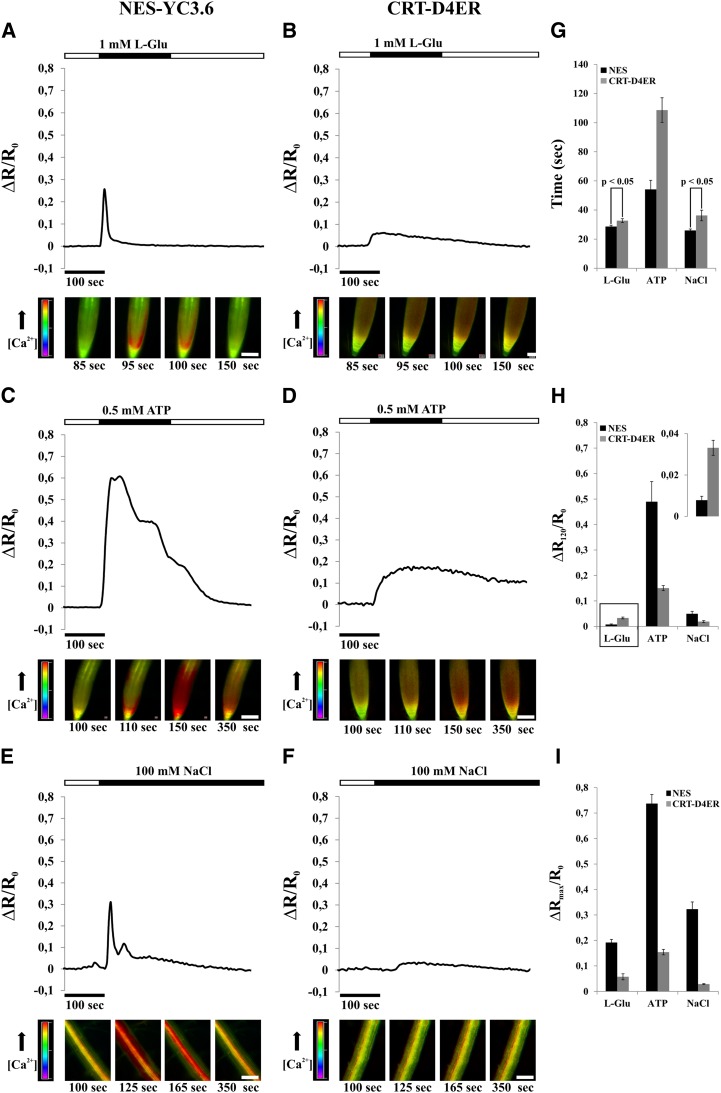
Subsequently, we sought to use CRT-D4ER to visualize potential variations in [Ca2+]ER in response to defined stimuli. To this end, we challenged 7-d-old Arabidopsis seedlings with different stimuli reported to induce cytosolic Ca2+ rises in root cells, such as external ATP (Tanaka et al., 2010; Loro et al., 2012, 2013), l-Glu (Qi et al., 2006), and NaCl (Kiegle et al., 2000). To allow for comparison with cytosolic Ca2+ dynamics and as a control for the efficiency of stimulus applications, we performed a series of parallel experiments using either seedlings expressing the cytosol-localized Cameleon YC3.6 (NES-YC3.6; Nagai et al., 2004; Krebs et al., 2012) or expressing CRT-D4ER (Fig. 2).
Figure 2.
[Ca2+]cyt and [Ca2+]ER monitoring in roots of Arabidopsis seedlings expressing the NES-YC3.6 (cytosolic Cameleon) and CRT-D4ER (ER Cameleon) probes, challenged with different stimuli (l-Glu, ATP, and NaCl) for the indicated times (black rectangles above traces) and analyzed with a wide-field fluorescence microscope. Traces represent the normalized ratio (FRET/CFP) variations observed during the entire experiment. Images at bottom are representative ratio images of the experiment shown in the corresponding trace above. A, l-Glu (1 mm) induced a single steep and fast Ca2+ transient in the cytosol of root tip cells. B, The same stimulus of A induced an ER Ca2+ accumulation showing a slow kinetic and a sustained Ca2+ recovery phase. C, ATP (0.5 mm) induced a strong cytosolic Ca2+ transient in the cytosol of root tip cells, where different peaks were recognizable. The recovery phase was more sustained compared with 1 mm l-Glu. D, The same stimulus of C induced an ER Ca2+ accumulation showing a slow kinetic, but with values about four times higher than those observed with 1 mm l-Glu and, afterward, with a small [Ca2+]ER decrease. E, NaCl (100 mm) induced a biphasic cytosolic Ca2+ response with two peaks of different intensities in cells of the root mature zone. F, The same salt concentration of E led to a small ER Ca2+ accumulation followed by a slow [Ca2+]ER decrease. G, Statistical analysis of times at which the maximum ΔR/R0 variations were measured in both compartments in response to different applied stimuli. H, Statistical analysis of ΔR/R0 variations measured in both compartments 120 s after sensing of the different applied stimuli. The inset shows a magnification of l-Glu Ca2+ peak averages. I, Statistical analysis of maximum ΔR/R0 variations measured in both compartments in response to different applied stimuli. P values were calculated using Student’s t test. Bars = 100 μm. [See online article for color version of this figure.]
One millimolar l-Glu was able to stimulate a fast and transient [Ca2+]cyt increase in root tip cells (Fig. 2, A and G). In particular, l-Glu triggered a response in the cells of the root tip region, and then Ca2+ elevations spread out quickly to the upper root tip cells (Fig. 2A; Supplemental Movies S1 and S2). The l-Glu-Ca2+-induced elevation is probably dependent on the activity of members of the ionotropic Glu receptors, channels that have been demonstrated to facilitate Ca2+ influx across the PM (Michard et al., 2011; Vincill et al., 2012, 2013). Several members of this large family (20 members in Arabidopsis; Lacombe et al., 2001) are in fact expressed in the different tissues of root tip, as reported by published microarray and experimental data (Winter et al., 2007; Vincill et al., 2013).
The same stimulus also triggered a concomitant increase of [Ca2+]ER (Fig. 2, B and G), which was more sustained compared with the rise observed in the cytosol (Fig. 2, B and H). Importantly, separate analyses of the two CFP and FRET fluorescences revealed an evident FRET response, manifested by a decrease of CFP and an increase of YFP emissions (Supplemental Fig. S2A).
A similar series of experiments was then performed with the addition of 0.5 mm ATP (Fig. 2, C and D). In agreement with previous reports, high extracellular ATP concentration resulted in a strong and sustained [Ca2+]cyt increase, with a typical dynamics consisting of different sequential Ca2+ peaks due to the influx of external Ca2+ and the release of Ca2+ from nonidentified internal stores (Fig. 2C; Supplemental Movie S3; Tanaka et al., 2010; Loro et al., 2012). This stimulus also induced an ER Ca2+ accumulation (Fig. 2D) in which the maximum level of [Ca2+]ER was reached later as compared with the cytosol (Fig. 2G) but was stronger and even more sustained as compared with the one induced by l-Glu (Fig. 2, D, H, and I). In none of these experiments did we observe a measurable reduction in [Ca2+]ER before the occurrence of the [Ca2+]cyt increase. To further address this aspect, a series of experiments was performed with 0.01 mm ATP, a concentration reported to stimulate ER Ca2+ release in HeLa cells (Palmer et al., 2004). As reported previously, root tip cells stimulated with 0.01 mm ATP displayed [Ca2+]cyt dynamics, albeit of lower magnitude (Supplemental Fig. S3, A and C–E; Loro et al., 2012). However, again, only an increase in [Ca2+]ER was observed (Supplemental Fig. S3, B–E). These results strongly support the conclusion that the ER does not represent the internal Ca2+-releasing store involved in the generation of the ATP-induced cytosolic Ca2+ dynamics. Finally, salt stress was analyzed as a third stimulus that is known to elicit an increase in cytosolic Ca2+ in root cells. The effect of NaCl was analyzed in the root mature zone, since these cells respond in a more pronounced manner than root tip cells to salt stress (data not shown). The addition of 100 mm NaCl induced a fast cytosolic Ca2+ increase (Fig. 2, E and G), which was accompanied by a Ca2+ accumulation in the ER (Fig. 2, F, H, and I). In the latter case, the source of cytosolic Ca2+ has been demonstrated to be both from extracellular and intracellular calcium stores, mainly from vacuole (Knight et al., 1997). Our results indeed demonstrate that the ER is not part of such intracellular stores. Moreover, it is worth noting that, compared with the response to l-Glu (Fig. 2, G–I), the increases in [Ca2+]cyt were similar in terms of peak intensities and duration, while the ER Ca2+ accumulation induced by NaCl treatment was smaller. This observation could be related to the fact that cells of the root mature zone have extensive vacuoles compared with the meristematic cells of the root tip, hence probably also contributing to Ca2+ sequestration (Peiter, 2011).
Altogether, these results indicate that plant cells transiently accumulate Ca2+ in the ER in response to different stimuli, similar to what was observed in the apoplast and mitochondria (Gao et al., 2004; Loro et al., 2012). The subsequent decrease in [Ca2+]ER, which is especially evident with l-Glu, low ATP concentration, and NaCl treatments, occurs only after the initial accumulation and is likely to be dependent on the activity of ER membrane-resident Ca2+-permeable channels (Klüsener et al., 1995, 1997; Muir and Sanders, 1997; Navazio et al., 2000, 2001). Importantly, these results suggest that, at least in the cases investigated here, the ER does not contribute to the generation of the observed cytosolic Ca2+ increases by releasing Ca2+. Instead, it appears that the ER represents a system that mimics [Ca2+]cyt increases, but with distinct dynamics, as marked by the different times at which the peaks of [Ca2+]cyt and [Ca2+]ER are reached after the stimuli perception (Fig. 2G). The delayed ER Ca2+ accumulation was particularly evident in ATP-stimulated cells (Fig. 2H; Supplemental Fig. S3E). These dynamics of ER Ca2+ accumulation may reflect the intrinsic properties of the ER-resident active Ca2+ transport systems (Bonza and De Michelis, 2011; Bose et al., 2011). Instead, the [Ca2+]cyt increase is mainly dependent on the activity of channels occurring at very much faster rate than transporters.
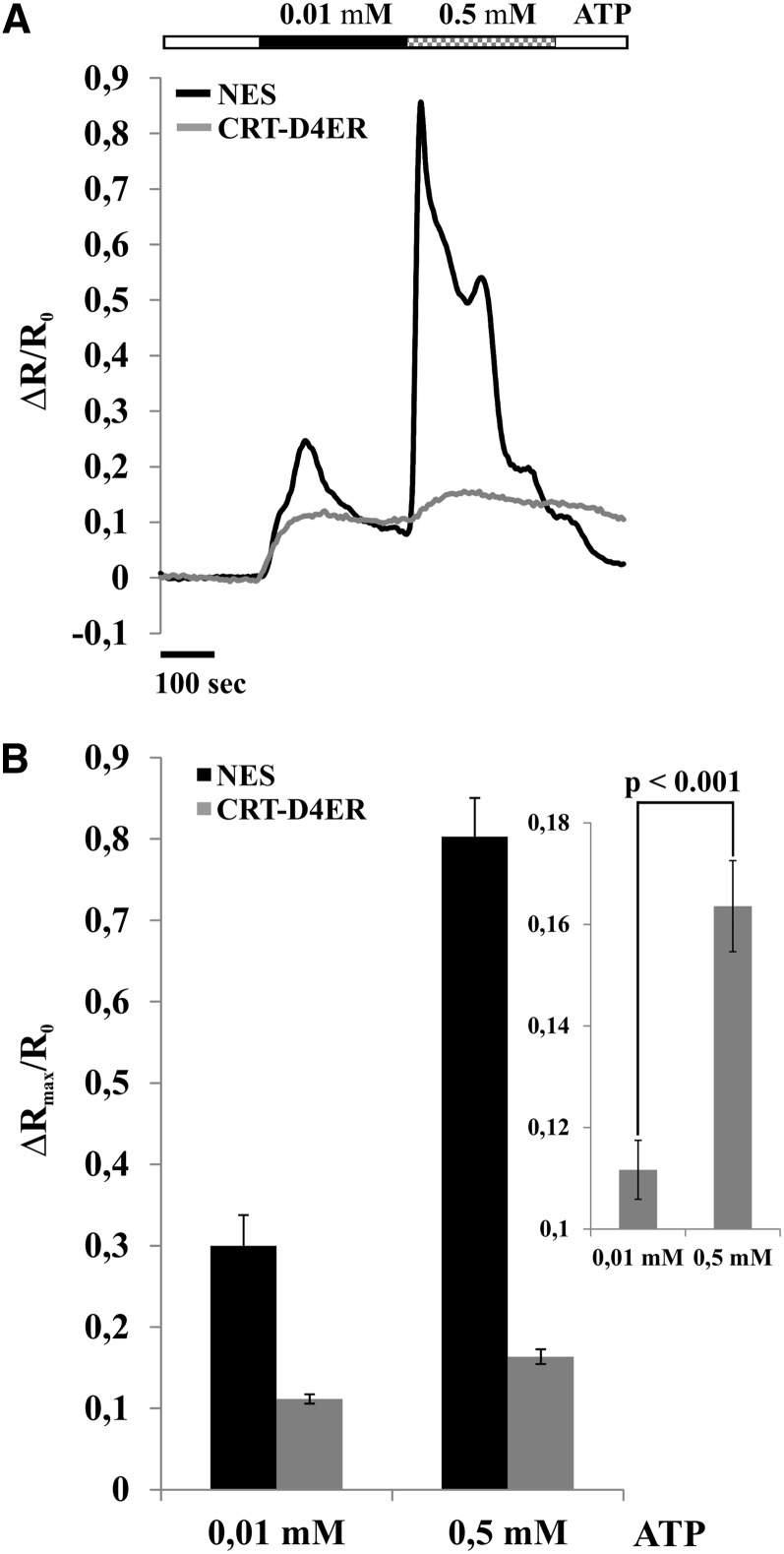
To corroborate that Ca2+ accumulation in the ER mimics cytosolic Ca2+ rises, we performed a series of experiments in which two different ATP concentrations were applied successively (0.01 mm followed by 0.5 mm) to root tip cells (Fig. 3). In particular, the second administration was applied before both [Ca2+]cyt and [Ca2+]ER were completely recovered to resting values. Figure 3A reports the superimposition of representative normalized ratio variations measured in the cytosol and ER, clearly showing that, in correspondence with each [Ca2+]cyt rise, a [Ca2+]ER rise occurs. Hence, the ER is able to accumulate Ca2+ at different levels in response to [Ca2+]cyt increases (Fig. 3B), supporting a role in buffering the cytosolic Ca2+ rises. Together, these results indicate that Ca2+ accumulation in the ER is strictly dependent on the [Ca2+]cyt.
Figure 3.
[Ca2+]cyt and [Ca2+]ER monitoring in root tips of Arabidopsis seedlings expressing the NES-YC3.6 (cytosolic Cameleon) and CRT-D4ER (ER Cameleon) probes challenged with 0.01 and 0.5 mm ATP for the indicated times (black and checkered rectangles above traces) and analyzed with a wide-field fluorescence microscope. A, Superimposition of representative normalized ratio variations for each transgenic line. B, Statistical analysis of maximum ΔR/R0 variations measured in both compartments. The inset shows a magnification of Ca2+ peak averages measured in the ER in response to 0.01 and 0.5 mm ATP. P values were calculated using Student’s t test.
Finally, when root cells were challenged with a series of hyperpolarizing-depolarizing buffers, known to induce repetitive cytosolic Ca2+ transients in guard and root cells (Allen et al., 2000, 2001; Weinl et al., 2008; Krebs et al., 2012), repetitive ER Ca2+ accumulations were also observed (Supplemental Fig. S4, A and B). Chelating extracellular Ca2+ with EGTA prevented both cytosolic and ER Ca2+ transients (Supplemental Fig. S4, C and D), confirming that the ER does not contribute as an intracellular Ca2+ store to the generation of stimulus-induced cytosolic Ca2+ rises observed. However, we can currently not fully exclude that localized Ca2+ releases from ER occur in specific microdomains of cells (Rizzuto and Pozzan, 2006). Based on our microscope resolution, the predominant response of a defined population of root tip cells was measurable that consequently represents the predominant component of ER Ca2+ dynamics in these cells.
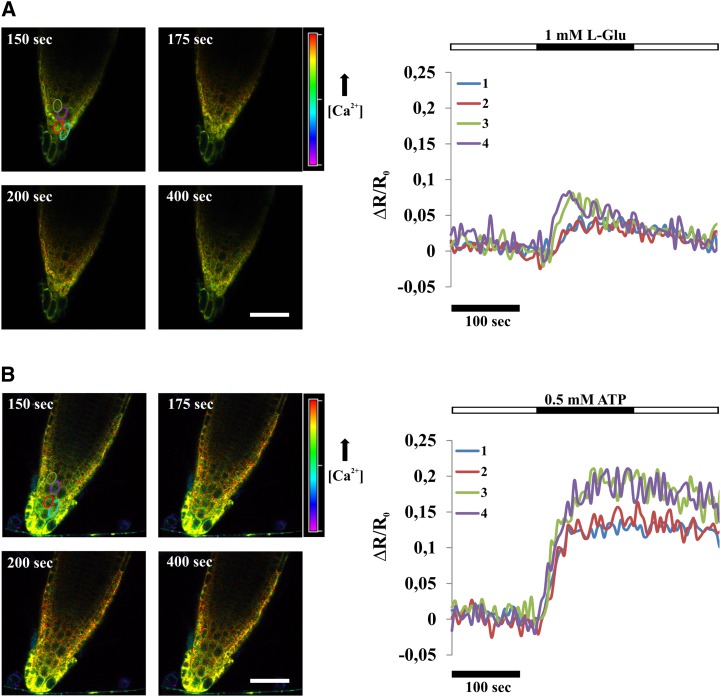
Comparison of ER Ca2+ dynamics induced in root tip cells by l-Glu and ATP revealed that Ca2+ accumulation in the ER was directly dependent on the magnitude of cytosolic Ca2+ elevation, with ATP inducing the strongest increase (compare the ΔR/R0 in Fig. 2, B, D, and I). In particular, the observed differences between the responses of root tip cells to l-Glu and ATP may result from a different number of cells responding primarily or secondarily to the stimulus (see the image sequences below the graphs in Fig. 2). In each single cell, the two stimuli may produce an increase in [Ca2+]ER of different amplitude. In order to appreciate Ca2+ dynamics in single cells, we performed an analysis of CRT-D4ER-expressing seedlings by means of confocal microscopy. The results, depicted in Figure 4 and Supplemental Movies S4 and S5, demonstrated that amplitudes and dynamics of ER Ca2+ transients induced by l-Glu and ATP in single cells essentially reflected the general root tip response. Importantly, these results confirm that our wide-field analyses faithfully reflect the cellular responsiveness of root tip cells to the stimuli investigated.
Figure 4.
Single-cell [Ca2+]ER monitoring in Arabidopsis CRT-D4ER seedling root tips subjected to l-Glu and ATP for the indicated times (black rectangles above traces). A, Effects of 1 mm l-Glu on the [Ca2+]ER response in Arabidopsis root tip cells. Ratio images for selected frames are shown. B, Effects of 0.5 mm ATP on the [Ca2+]ER response in Arabidopsis root tip cells. Ratio images for selected frames are shown. In both panels, elliptic areas, marked with different colors corresponding to different cells, were used for the ratio calculations (reported as normalized ratio variations [ΔR/R0]) plotted in the graphs. The two different stimuli triggered responses of different amplitudes, with ATP able to induce higher ER Ca2+ accumulations in all analyzed cells compared with l-Glu. Bars = 50 μm.
Altogether, these experiments demonstrate that the CRT-D4ER represents a reliable probe to monitor ER Ca2+ dynamics in vivo at both the organ and single-cell levels. Moreover, our results suggest that, in the cells investigated under the conditions examined here, the ER does not represent a major store for releasing Ca2+ into the cytosol.
ER-Localized Ca2+-ATPases Modulate ER Ca2+ Homeostasis
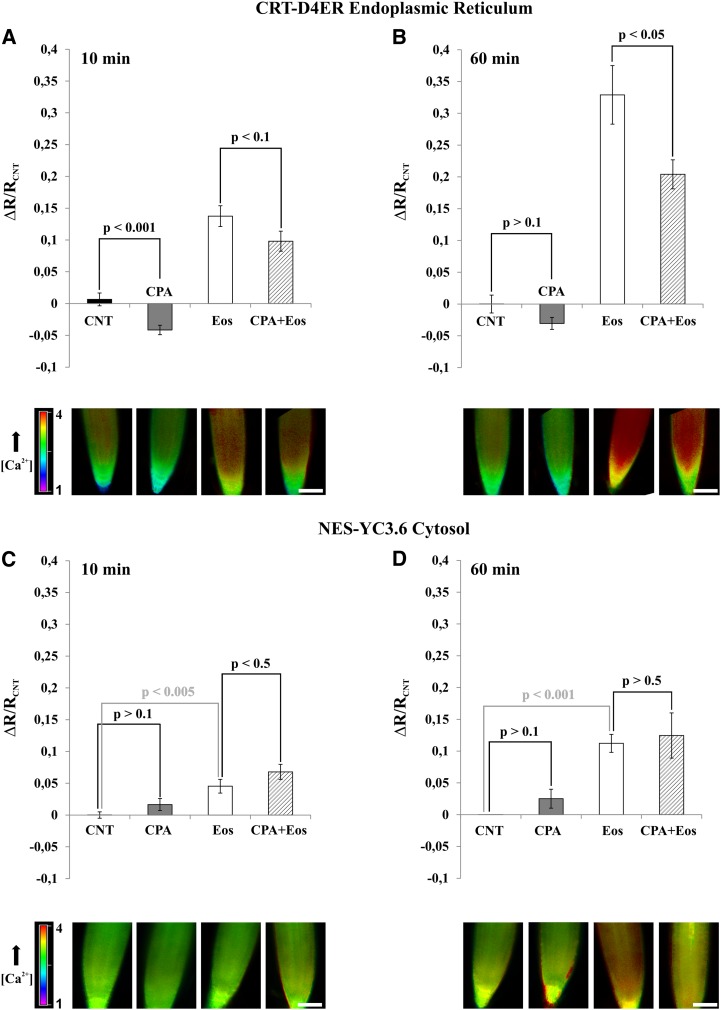
Having established that dynamic changes in Ca2+ accumulation occur in the ER in response to defined stimuli, we next aimed at elucidating the contribution of ER-resident Ca2+-ATPases to [Ca2+]ER homeostasis. In Arabidopsis, members of both classes of Ca2+ pumps are resident in the ER membrane (Sze et al., 2000; Bonza and De Michelis, 2011). ECAs are specifically inhibited by cyclopiazonic acid (CPA; Liang and Sze, 1998), while ACAs are particularly sensitive to inhibition by fluorescein derivatives such as erythrosin B or eosin Y (Eos; De Michelis et al., 1993; Geisler et al., 2000; Sze et al., 2000; Bonza et al., 2004; Bonza and De Michelis, 2011). Therefore, in order to evaluate the contribution of the different types of Ca2+-ATPases to ER Ca2+ homeostasis, a pharmacological approach was pursued. Published microarray data (Winter et al., 2007) confirmed that root tips of young Arabidopsis seedlings express Ca2+ pumps of both types at various cellular membranes, including the ER-localized Ca2+-ATPase isoforms ACA2 and ECA1 (data not shown).
Arabidopsis seedlings expressing the CRT-D4ER or NES-YC3.6 Cameleon reporter protein were incubated for 10 or 60 min with or without 25 μm CPA, 0.5 μm Eos, and 25 μm CPA plus 0.5 μm Eos. In order to evaluate the [Ca2+]ER and [Ca2+]cyt levels in the different tested conditions, root tips were then analyzed by measuring the ratio values for both CRT-D4ER and NES-YC3.6 probes (Fig. 5). Root tip cells were chosen for the analysis because they harbor smaller vacuoles compared with cells of the mature zone. In this way, we probably reduced the relative contribution of the vacuolar Ca2+ transport systems to the regulation of cytosolic Ca2+ homeostasis (Cheng et al., 2005; Conn et al., 2011; Peiter, 2011).
Figure 5.
Effects of Ca2+-ATPase inhibitors on [Ca2+]cyt and [Ca2+]ER in root tip cells of Arabidopsis. Seedlings expressing NES-YC3.6 and CRT-D4ER were incubated for 10 and 60 min in control buffer (see “Materials and Methods”) or in the presence of 25 μm CPA, 0.5 μm Eos, and 25 μm CPA + 0.5 μm Eos. Root tips were analyzed with a wide-field fluorescence microscope. Bars represent the averaged normalized ratios (ΔR/RCNT) ± se. The ratios were normalized to the average ratio (RCNT) of each control experiment. The images at bottom are representative ratio images of each tested condition. A and B, [Ca2+]ER Cameleon ratio variations measured in root tip of CRT-D4ER seedlings treated for 10 and 60 min, respectively, with the different inhibitors. C and D, [Ca2+]cyt Cameleon ratio variations measured in root tip of NES-YC3.6 seedlings treated for 10 and 60 min, respectively, with the different inhibitors. P values were calculated using Student’s t test. Bars = 100 μm.[See online article for color version of this figure.]
The ECA inhibitor CPA affected the CRT-D4ER ratio already after 10 min of treatment, resulting in a significant decrease in the [Ca2+]ER compared with the control condition (Fig. 5A, gray bar). Surprisingly, treatment with the ACA inhibitor Eos did not lead to a [Ca2+]ER decrease but to a [Ca2+]ER increase (Fig. 5A, white bar). Interestingly, when treatments were performed with both inhibitors simultaneously (Fig. 5A, striped bar), the [Ca2+]ER was lower than in the Eos treatment alone but higher than with CPA alone. This suggests that the CPA-sensitive component is just partially responsible for the observed Eos-induced ER Ca2+ accumulation. After 60 min of incubation, the results were quite similar with CPA (Fig. 5B, gray bar), while the Eos effect on [Ca2+]ER increases was dramatically enhanced (Fig. 5B, white bar). In the latter condition, the presence of CPA was still able to reduce the [Ca2+]ER (Fig. 5B, striped bar). When the effect of inhibitors was tested on NES-YC3.6 seedlings, we observed that treatment with CPA for 10 min barely affected the [Ca2+]cyt (Fig. 5C, gray bar), while treatment with Eos led to an increase of [Ca2+]cyt (Fig. 5C, gray bar) that was further slightly increased by the simultaneous addition of CPA (Fig. 5C, striped bar). Extension of the treatment to 60 min caused similar, but more pronounced, results.
In summary, the combined analyses of Ca2+ dynamics in ER and cytosol with Ca2+-ATPase inhibitors revealed that, under basal conditions, CPA-sensitive ECAs substantially contribute to ER Ca2+ accumulation but have a minor role in the control of [Ca2+]cyt levels. Application of Eos compromised the activity of the ACA pumps that are fundamental for the maintenance of basal [Ca2+]cyt in resting conditions but did not prevent the accumulation of Ca2+ in the ER, which actually increased. This increase was reduced, but not fully suppressed, by CPA. Considering that under the applied conditions CPA suppressed ECA activity, these results indicate that, under elevated [Ca2+]cyt, ECAs contribute to [Ca2+]ER accumulation, but other transport systems are also involved. We cannot exclude that the Eos concentration used in this study was sufficient to inhibit ACA localized at cellular membranes such as PM and tonoplast (Bonza and De Michelis, 2011; Bose et al., 2011) but not fully effective in completely blocking the activity of the ER-resident ACAs (e.g. ACA2; Harper et al., 1998). Hence, it is plausible that the observed elevation of cytosolic Ca2+, due to the inhibition of cytosolic Ca2+ removal, leads to higher ER Ca2+ accumulation.
CONCLUSION
In this work, we report (1) the generation of the CRT-D4ER Cameleon reporter protein as a suitable tool for the analysis of Ca2+ status and dynamics in the ER of plant cells; (2) the analysis of ER Ca2+ dynamics in root tip cells in response to defined stimuli; and (3) the evaluation of the contribution of ECA and ACA Ca2+-ATPases to the control of ER Ca2+ homeostasis.
The application of CRT-D4ER enables the study of the dynamics of [Ca2+]ER and its interconnection with cytosolic Ca2+ signatures in different cell types, genetic backgrounds, and developmental and stress-response processes.
Using this tool, we comparatively analyzed the dynamics of [Ca2+]ER and [Ca2+]cyt in response to different stimuli like ATP, l-Glu, NaCl, and alternate applications of depolarizing and hyperpolarizing buffer. In conclusion, all our data do not support a significant role of [Ca2+]ER as a source of Ca2+ release that contributes to the formation of cytosolic Ca2+ signatures, at least in the cell types and during the responses investigated in this study. Instead, our data support the hypothesis that, in plant cells, the ER functions primarily as a mimicking system for cytosolic Ca2+ signaling. These findings point to fundamental differences in the role of the ER for cellular Ca2+ dynamics in plants and animals.
MATERIALS AND METHODS
Plant Material and Growth Conditions
All Arabidopsis (Arabidopsis thaliana) plants used in this study were of the Columbia ecotype. Plants were grown on 16/8-h cycles of light (70 μmol m−2 s−1) at 22°C and 75% relative humidity. Seeds of Arabidopsis were surface sterilized by vapor-phase sterilization (Clough and Bent, 1998) and plated on one-half Murashige and Skoog medium (Murashige and Skoog medium plus M0222 elements including vitamins; Duchefa [http://www.duchefa-biochemie.nl/]; Murashige and Skoog, 1962) supplemented with 0.1% (w/v) Suc and 0.05% (w/v) MES, pH 6.0, and solidified with 0.8% (w/v) plant agar (Duchefa). After stratification at 4°C in the dark for 3 d, seeds were transferred to the growth chamber with 16/8-h cycles of light (70 μmol m−2 s−1) at 24°C. The plates were kept vertically. Seedlings used for the analyses were 7 to 8 d old, which corresponds to an average root length of 3 cm.
DNA Constructs
In order to generate the binary vectors for the expression of the Cameleon ER probes in plants, we inserted the single CaMV 35S promoter and the CaMV poly(A) terminator in the polylinker of the pGreen0029 binary vector (Hellens et al., 2000) by using the KpnI and SacI restriction sites, respectively. The CaMV 35S and CaMV poly(A) were PCR amplified using the 35S-CaMV cassette vector as a template (http://www.pgreen.ac.uk/JIT/JIT_fr.htm) and the following primer pairs: 35S-For, 5′-CATGggtaccGATATCGTACCCCTACTCCA-3′; 35S-Rev, 5′-CATGggtaccGGGCTGTCCTCTCCAAATGAA-3′; Ter-For, 5′-CATGgagctcGGTACGCTGAAATCACCAGT-3′; and Ter-Rev, 5′-CATGgagctcATCGATCTGGATTTTAGTACTGGA-3′ (the sequences of the restriction sites are shown in lower-case letters). D1ER (AY796115.1) and D4ER (Plasmid 37473: pBAD-D4; http://www.addgene.org/37473/) coding sequences were digested from the pcDNA3-D1ER and pcDNA3-D4ER vectors with HindIII and EcoRI restriction enzymes and ligated into the modified pGreen0029-35S-Ter binary vector. In order to generate the CRT-D1ER and CRT-D4ER constructs, the first 66 nucleotides (CRT) of the Arabidopsis CALRETICULIN1A gene (At1g56340) were PCR amplified and fused upstream the D1ER and D4ER coding sequences by using the HindIII restriction site. The primers used were as follows: CRT-For, 5′-CATGaagcttATGGCGAAACTAAACCCTAAATT-3′; and CRT-Rev, 5′-CATGaagcttgAGCAGAGACGATCACCACGA-3′. The amplicon was isolated by digestion and ligated into the 35S-D1ER and 35S-D4ER linearized vectors. The obtained clones were sequenced to verify the right orientation of the CRT sequence.
The binary vectors were then introduced into the Agrobacterium tumefaciens GV3101 strain.
Transgenic Plants and Tobacco Transient Transformation
The A. tumefaciens strains obtained as reported above were used to generate transgenic Arabidopsis plants by the floral dip method (Clough and Bent, 1998). For each construct, 15 Arabidopsis independent transgenic lines were selected, and four independent lines were employed for imaging experiments. Experiments were carried out in seedlings of the T1 and T2 generations for CRT-D4ER transgenic lines. Mature T2 plants were affected by silencing. To minimize this problem, work is in progress to test different vector backbones and promoters.
Transient expression of CRT-D4ER in tobacco (Nicotiana benthamiana) epidermal leaf cells was performed as described by Waadt and Kudla (2008).
Seedling Preparation for Ca2+ Imaging
For root cell imaging, 7-d-old seedlings grown vertically were prepared accordingly to Behera and Kudla (2013) in dedicated chambers and overlaid with wet cotton in order to continuously perfuse the root with the imaging solution (5 mm KCl, 10 mm MES, 10 mm Ca2+, pH 5.8, adjusted with Tris for ATP and l-Glu, or 0.1 mm KCl, 10 mm MES, 1 mm Ca2+, pH 5.8, adjusted with Tris for NaCl). The shoot was not submerged in the solution. l-Glu and ATP were added as disodium or magnesium salt, respectively, to the chamber by perfusion with the same solution. For chemical treatments, seedlings were preincubated for 10 or 60 min in 5-cm petri dishes in the imaging solution supplemented with 25 μm CPA, 0.5 μm Eos, or a combination of both. Control seedlings were kept for the same times in the imaging solution supplemented with 0.25% (v/v) dimethyl sulfoxide. Seedlings were then transferred to the imaging chamber and allowed to recover for approximately 7 min prior to measurement.
Time-Lapse Ca2+ Imaging and Confocal Microscopy Analyses
Cameleon seedling roots and leaves were imaged in vivo by an inverted fluorescence Nikon microscope (Ti-E; http://www.nikon.com/) with a CFI planfluor 4× numerical aperture 0.13 dry objective and a CFI PLAN APO 20× VC dry objective. Excitation light was produced by a fluorescent lamp (Prior Lumen 200 PRO; Prior Scientific; http://www.prior.com) at 440 nm (436/20 nm) set to 20%. Images were collected with a Hamamatsu Dual CCD camera (ORCA-D2; http://www.hamamatsu.com/). For Cameleon analysis, the FRET CFP/YFP optical block A11400-03 (emission 1, 483/32 nm for CFP; emission 2, 542/27 nm for FRET) with a dichroic 510-nm mirror (Hamamatsu) was used for the simultaneous CFP and FRET acquisitions (citrine for D1, YFP for D4, and cpVenus for YC3.6). Exposure time was from 100 to 400 ms with a 2 × 2 CCD binning for cytosolic Cameleon (NES-YC3.6) and a 4 × 4 CCD binning for ER Cameleons (CRT-D1ER and CRT-D4ER). Images where acquired every 5 s. Filters and the dichroic mirror were purchased from Chroma Technology (http://www.chroma.com/). NIS-Elements (Nikon; http://www.nis-elements.com/) was used as a platform to control microscope, illuminator, camera, and postacquisition analyses.
With regard to time-course experiments, fluorescence intensity was determined over regions of interest that correspond to the root tip zone. FRET and CFP emissions of the analyzed regions of interest were used for the ratio (R) calculation (FRET/CFP) and normalized to the initial ratio (R0) and plotted versus time (ΔR/R0). The background was independently subtracted for both channels before calculating the ratio.
Confocal microscopy analyses were performed using Nikon C2 (http://www.nikoninstruments.com) and Leica SP5 (http://www.leica-microsystems.com) laser scanning confocal imaging systems. For Cameleon-dependent citrine (D1), YFP (D4), and cpVenus (YC3.6), excitation was at 488 nm and emission was between 525 and 540 nm. For mCherry detection, excitation was at 561 nm and emission was between 590 and 620 nm. Image analyses were done with the ImageJ bundle software (http://rsb.info.nih.gov/ij/).
For Ca2+ imaging analyses, roots were imaged with a 63× lens (H 63X PL APO numerical aperture 1.20 water immersion), and the Cameleons were excited with the 458-nm line of the argon laser with 15% of total power. The CFP and FRET emissions were collected at 473 to 505 nm and 526 to 536 nm, respectively, and the pinhole diameter was 2 to 4 airy units depending on the line used. Images were collected every 5 s. The false color ratio images were obtained by using the NIS-Elements (Nikon; http://www.nis-elements.com/). For time-course experiments, fluorescence intensity was determined over regions of interest that correspond to single cells in the root tip zone. FRET and CFP emissions of the analyzed regions of interest were used for the ratio (R) calculation (FRET/CFP) and normalized to the initial ratio (R0) and plotted versus time (ΔR/R0).
All the data are representative of at least six independent cells or roots analyzed unless otherwise stated. Reported traces are representative ones chosen from a set of six or more identical experiments, and the data shown as bar diagrams are averages from corresponding data sets. Data are reported as averages ± se, and statistical significance was calculated by Student’s t test.
In Vivo Dynamic Range of CRT-D4ER
In order to determine the in vivo dynamic range of the CRT-D4ER probe (ΔRmax/R0), we considered the Rmin and Rmax measured in the experiments performed in root tip seedlings incubated with Ca2+-ATPase inhibitors. The measured Rmin was 2.71 ± 0.021 in the presence of 25 μm CPA after 10 min of incubation, whereas the Rmax was 3.78 ± 0.18 in the presence of 0.5 μm Eos for 60 min. Hence, the calculated ΔRmax/R = 0.395 shows that the responses to the different observed stimuli did not lead to a probe saturation.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Subcellular distribution of CRT-D1ER in transgenic Arabidopsis plants and simultaneous detection of CFP and FRET emissions.
Supplemental Figure S2. FRET detection in Arabidopsis seedlings expressing the CRT-D4ER (ER Cameleon) probe challenged with 1 mm l-Glu, 0.1 mm ATP, and 100 mm NaCl.
Supplemental Figure S3. [Ca2+]cyt and [Ca2+]ER dynamics in root cells of Arabidopsis seedlings expressing the NES-YC3.6 (cytosolic Cameleon) and CRT-D4ER (ER Cameleon) probes challenged with 0.01 mm ATP.
Supplemental Figure S4. [Ca2+]cyt and [Ca2+]ER dynamics in root cells of Arabidopsis seedlings expressing the NES-YC3.6 (cytosolic Cameleon) and CRT-D4ER (ER Cameleon) probes challenged with repetitive depolarizing-hyperpolarizing buffer changes in the presence or absence of EGTA.
Supplemental Text S1. Generation and analysis of Arabidopsis plants expressing the CRT-D1ER Cameleon probe.
Supplemental Movie S1. Series of cytosolic Ca2+ ratio images (low magnification, 4×) of an Arabidopsis seedling root tip expressing NES-YC3.6 challenged with 1 mm l-Glu.
Supplemental Movie S2. Series of cytosolic Ca2+ ratio images of an Arabidopsis seedling root tip expressing NES-YC3.6 challenged with 1 mm l-Glu analyzed by confocal laser scanning microscopy.
Supplemental Movie S3. Series of cytosolic Ca2+ ratio images of an Arabidopsis seedling root tip expressing NES-YC3.6 challenged with 0.5 mm ATP analyzed by confocal laser scanning microscopy.
Supplemental Movie S4. Series of ER Ca2+ ratio images of an Arabidopsis seedling root tip expressing CRT-D4ER challenged with 1 mm l-Glu analyzed by confocal laser scanning microscopy.
Supplemental Movie S5. Series of ER Ca2+ ratio images of an Arabidopsis seedling root tip expressing CRT-D4ER challenged with 0.5 mm ATP analyzed by confocal laser scanning microscopy.
Acknowledgments
We thank Maria Ida De Michelis (University of Milan) for critical reading of the manuscript; Prof. Anna Moroni (University of Milan) for helpful discussions; Karin Schumacher (University of Heidelberg) for providing us the UBQ10-NES-YC3.6 Arabidopsis plants; Roger Tsien (University of California, San Diego) for providing us the pCDNA3-D1ER vector; and Tullio Pozzan (University of Padova) for the pCDNA3-D1ER vector.
Glossary
- [Ca2+]cyt
cytosolic Ca2+ concentration
- PM
plasma membrane
- FRET
fluorescence resonance energy transfer
- CFP
cyan fluorescent protein
- YFP
yellow fluorescent protein
- CaM
calmodulin
- ER
endoplasmic reticulum
- SOCE
store-operated Ca2+ entry
- IP3
inositol 1,4,5-trisphosphate
- [Ca2+]ER
endoplasmic reticulum Ca2+ concentration
- CaMV
cauliflower mosaic virus
- CPA
cyclopiazonic acid
- Eos
eosin Y
References
- Allen GJ, Kwak JM, Chu SP, Llopis J, Tsien RY, Harper JF, Schroeder JI. (1999) Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J 19: 735–747 [DOI] [PubMed] [Google Scholar]
- Allen GJ, Chu SP, Schumacher K, Shimazaki CT, Vafeados D, Kemper A, Hawke SD, Tallman G, Tsien RY, Harper JF, et al (2000) Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289: 2338–2342 [DOI] [PubMed] [Google Scholar]
- Allen GJ, Chu SP, Harrington CL, Schumacher K, Hoffmann T, Tang YY, Grill E, Schroeder JI. (2001) A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 411: 1053–1057 [DOI] [PubMed] [Google Scholar]
- Baxter I, Tchieu J, Sussman MR, Boutry M, Palmgren MG, Gribskov M, Harper JF, Axelsen KB. (2003) Genomic comparison of P-type ATPase ion pumps in Arabidopsis and rice. Plant Physiol 132: 618–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behera S, Krebs M, Loro G, Schumacher K, Costa A, Kudla J. (2013) Ca2+ imaging in plants using genetically encoded Yellow Cameleon Ca2+ indicators. Cold Spring Harb Protoc 2013: 700–703 [DOI] [PubMed] [Google Scholar]
- Behera S, Kudla J. (2013) High-resolution imaging of cytoplasmic Ca2+ dynamics in Arabidopsis roots. Cold Spring Harb Protoc 2013: 665–669 [DOI] [PubMed] [Google Scholar]
- Berridge MJ. (2009) Inositol trisphosphate and calcium signalling mechanisms. Biochim Biophys Acta 1793: 933–940 [DOI] [PubMed] [Google Scholar]
- Boevink P, Martin B, Oparka K, Santa Cruz S, Hawes C. (1999) Transport of virally expressed green fluorescent protein through the secretory pathway in tobacco leaves is inhibited by cold shock and brefeldin A. Planta 208: 392–400 [Google Scholar]
- Bonza MC, De Michelis MI. (2011) The plant Ca2+-ATPase repertoire: biochemical features and physiological functions. Plant Biol (Stuttg) 13: 421–430 [DOI] [PubMed] [Google Scholar]
- Bonza MC, Luoni L, De Michelis MI. (2004) Functional expression in yeast of an N-deleted form of At-ACA8, a plasma membrane Ca2+-ATPase of Arabidopsis thaliana, and characterization of a hyperactive mutant. Planta 218: 814–823 [DOI] [PubMed] [Google Scholar]
- Bose J, Pottosin II, Shabala SS, Palmgren MG, Shabala S. (2011) Calcium efflux systems in stress signaling and adaptation in plants. Front Plant Sci 2: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandizzi F, Fricker M, Hawes C. (2002) A greener world: the revolution in plant bioimaging. Nat Rev Mol Cell Biol 3: 520–530 [DOI] [PubMed] [Google Scholar]
- Carrasco S, Meyer T. (2010) Cracking CRAC. Nat Cell Biol 12: 416–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng NH, Pittman JK, Shigaki T, Lachmansingh J, LeClere S, Lahner B, Salt DE, Hirschi KD. (2005) Functional association of Arabidopsis CAX1 and CAX3 is required for normal growth and ion homeostasis. Plant Physiol 138: 2048–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen A, Svensson K, Thelin L, Zhang W, Tintor N, Prins D, Funke N, Michalak M, Schulze-Lefert P, Saijo Y, et al. (2010) Higher plant calreticulins have acquired specialized functions in Arabidopsis. PLoS ONE 5: e11342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Coe H, Michalak M. (2009) Calcium binding chaperones of the endoplasmic reticulum. General Physiology and Biophysics 28: F96–F103 [PubMed] [Google Scholar]
- Collins SR, Meyer T. (2011) Evolutionary origins of STIM1 and STIM2 within ancient Ca2+ signaling systems. Trends Cell Biol 21: 202–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn SJ, Gilliham M, Athman A, Schreiber AW, Baumann U, Moller I, Cheng NH, Stancombe MA, Hirschi KD, Webb AA, et al. (2011) Cell-specific vacuolar calcium storage mediated by CAX1 regulates apoplastic calcium concentration, gas exchange, and plant productivity in Arabidopsis. Plant Cell 23: 240–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett EF, Michalak M. (2000) Calcium, a signaling molecule in the endoplasmic reticulum? Trends Biochem Sci 25: 307–311 [DOI] [PubMed] [Google Scholar]
- Costa A, Drago I, Behera S, Zottini M, Pizzo P, Schroeder JI, Pozzan T, Lo Schiavo F. (2010) H2O2 in plant peroxisomes: an in vivo analysis uncovers a Ca2+-dependent scavenging system. Plant J 62: 760–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Drago I, Zottini M, Pizzo P, Pozzan T. (2013) Peroxisome Ca2+ homeostasis in animal and plant cells. Subcell Biochem 69: 111–133 [DOI] [PubMed] [Google Scholar]
- De Michelis MI, Carnelli A, Rasi-Caldogno F. (1993) The Ca2+ pump of the plasma membrane of Arabidopsis thaliana: characteristics and sensitivity to fluorescein derivatives. Bot Acta 106: 20–25 [Google Scholar]
- Dodd AN, Kudla J, Sanders D. (2010) The language of calcium signaling. Annu Rev Plant Biol 61: 593–620 [DOI] [PubMed] [Google Scholar]
- Feske S, Skolnik EY, Prakriya M. (2012) Ion channels and transporters in lymphocyte function and immunity. Nat Rev Immunol 12: 532–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler M, Axelsen KB, Harper JF, Palmgren MG. (2000) Molecular aspects of higher plant P-type Ca2+-ATPases. Biochim Biophys Acta 1465: 52–78 [DOI] [PubMed] [Google Scholar]
- Galione A, Chuang KT. (2012) Pyridine nucleotide metabolites and calcium release from intracellular stores. Adv Exp Med Biol 740: 305–323 [DOI] [PubMed] [Google Scholar]
- Gao D, Knight MR, Trewavas AJ, Sattelmacher B, Plieth C. (2004) Self-reporting Arabidopsis expressing pH and [Ca2+] indicators unveil ion dynamics in the cytoplasm and in the apoplast under abiotic stress. Plant Physiol 134: 898–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JF, Hong B, Hwang I, Guo HQ, Stoddard R, Huang JF, Palmgren MG, Sze H. (1998) A novel calmodulin-regulated Ca2+-ATPase (ACA2) from Arabidopsis with an N-terminal autoinhibitory domain. J Biol Chem 273: 1099–1106 [DOI] [PubMed] [Google Scholar]
- Hawes C, Saint-Jore C, Martin B, Zheng HQ. (2001) ER confirmed as the location of mystery organelles in Arabidopsis plants expressing GFP! Trends Plant Sci 6: 245–246 [DOI] [PubMed] [Google Scholar]
- Hellens RP, Edwards EA, Leyland NR, Bean S, Mullineaux PM. (2000) pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol Biol 42: 819–832 [DOI] [PubMed] [Google Scholar]
- Hetherington AM, Brownlee C. (2004) The generation of Ca2+ signals in plants. Annu Rev Plant Biol 55: 401–427 [DOI] [PubMed] [Google Scholar]
- Hong B, Ichida A, Wang Y, Gens JS, Pickard BG, Harper JF. (1999) Identification of a calmodulin-regulated Ca2+-ATPase in the endoplasmic reticulum. Plant Physiol 119: 1165–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Sze H, Harper JF. (2000) A calcium-dependent protein kinase can inhibit a calmodulin-stimulated Ca2+ pump (ACA2) located in the endoplasmic reticulum of Arabidopsis. Proc Natl Acad Sci USA 97: 6224–6229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwano M, Entani T, Shiba H, Kakita M, Nagai T, Mizuno H, Miyawaki A, Shoji T, Kubo K, Isogai A, et al (2009) Fine-tuning of the cytoplasmic Ca2+ concentration is essential for pollen tube growth. Plant Physiol 150: 1322–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Moreno R, Wang ZM, Messi ML, Delbono O. (2010) Sarcoplasmic reticulum Ca2+ depletion in adult skeletal muscle fibres measured with the biosensor D1ER. Pflugers Arch 459: 725–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiegle E, Moore CA, Haseloff J, Tester MA, Knight MR. (2000) Cell-type-specific calcium responses to drought, salt and cold in the Arabidopsis root. Plant J 23: 267–278 [DOI] [PubMed] [Google Scholar]
- Kipanyula MJ, Contreras L, Zampese E, Lazzari C, Wong AK, Pizzo P, Fasolato C, Pozzan T. (2012) Ca2+ dysregulation in neurons from transgenic mice expressing mutant presenilin 2. Aging Cell 11: 885–893 [DOI] [PubMed] [Google Scholar]
- Klüsener B, Boheim G, Liss H, Engelberth J, Weiler EW. (1995) Gadolinium-sensitive, voltage-dependent calcium release channels in the endoplasmic reticulum of a higher plant mechanoreceptor organ. EMBO J 14: 2708–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klüsener B, Boheim G, Weiler EW. (1997) Modulation of the ER Ca2+ channel BCC1 from tendrils of Bryonia dioica by divalent cations, protons and H2O2. FEBS Lett 407: 230–234 [DOI] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR. (1997) Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J 12: 1067–1078 [DOI] [PubMed] [Google Scholar]
- Krebs M, Held K, Binder A, Hashimoto K, Den Herder G, Parniske M, Kudla J, Schumacher K. (2012) FRET-based genetically encoded sensors allow high-resolution live cell imaging of Ca²⁺ dynamics. Plant J 69: 181–192 [DOI] [PubMed] [Google Scholar]
- Kudla J, Batistic O, Hashimoto K. (2010) Calcium signals: the lead currency of plant information processing. Plant Cell 22: 541–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe B, Becker D, Hedrich R, DeSalle R, Hollmann M, Kwak JM, Schroeder JI, Le Novère N, Nam HG, Spalding EP, et al. (2001) The identity of plant glutamate receptors. Science 292: 1486–1487 [DOI] [PubMed] [Google Scholar]
- Liang F, Cunningham KW, Harper JF, Sze H. (1997) ECA1 complements yeast mutants defective in Ca2+ pumps and encodes an endoplasmic reticulum-type Ca2+-ATPase in Arabidopsis thaliana. Proc Natl Acad Sci USA 94: 8579–8584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F, Sze H. (1998) A high-affinity Ca2+ pump, ECA1, from the endoplasmic reticulum is inhibited by cyclopiazonic acid but not by thapsigargin. Plant Physiol 118: 817–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loro G, Drago I, Pozzan T, Schiavo FL, Zottini M, Costa A. (2012) Targeting of Cameleons to various subcellular compartments reveals a strict cytoplasmic/mitochondrial Ca2+ handling relationship in plant cells. Plant J 71: 1–13 [DOI] [PubMed] [Google Scholar]
- Loro G, Ruberti C, Zottini M, Costa A. (2013) The D3cpv Cameleon reports Ca2+ dynamics in plant mitochondria with similar kinetics of the YC3.6 Cameleon, but with a lower sensitivity. J Microsc 249: 8–12 [DOI] [PubMed] [Google Scholar]
- Luciani DS, Gwiazda KS, Yang TL, Kalynyak TB, Bychkivska Y, Frey MH, Jeffrey KD, Sampaio AV, Underhill TM, Johnson JD. (2009) Roles of IP3R and RyR Ca2+ channels in endoplasmic reticulum stress and beta-cell death. Diabetes 58: 422–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima R, Hayashi Y, Kondo M, Shimada T, Nishimura M, Hara-Nishimura I. (2002) An endoplasmic reticulum-derived structure that is induced under stress conditions in Arabidopsis. Plant Physiol 130: 1807–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima R, Kondo M, Nishimura M, Hara-Nishimura I. (2003) A novel ER-derived compartment, the ER body, selectively accumulates a beta-glucosidase with an ER-retention signal in Arabidopsis. Plant J 33: 493–502 [DOI] [PubMed] [Google Scholar]
- McAinsh MR, Pittman JK. (2009) Shaping the calcium signature. New Phytol 181: 275–294 [DOI] [PubMed] [Google Scholar]
- Michard E, Lima PT, Borges F, Silva AC, Portes MT, Carvalho JE, Gilliham M, Liu LH, Obermeyer G, Feijó JA. (2011) Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil D-serine. Science 332: 434–437 [DOI] [PubMed] [Google Scholar]
- Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. (1997) Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 388: 882–887 [DOI] [PubMed] [Google Scholar]
- Monshausen GB, Messerli MA, Gilroy S. (2008) Imaging of the Yellow Cameleon 3.6 indicator reveals that elevations in cytosolic Ca2+ follow oscillating increases in growth in root hairs of Arabidopsis. Plant Physiol 147: 1690–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir SR, Sanders D. (1997) Inositol 1,4,5-trisphosphate-sensitive Ca2+ release across nonvacuolar membranes in cauliflower. Plant Physiol 114: 1511–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Nagai T, Yamada S, Tominaga T, Ichikawa M, Miyawaki A. (2004) Expanded dynamic range of fluorescent indicators for Ca2+ by circularly permuted yellow fluorescent proteins. Proc Natl Acad Sci USA 101: 10554–10559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navazio L, Bewell MA, Siddiqua A, Dickinson GD, Galione A, Sanders D. (2000) Calcium release from the endoplasmic reticulum of higher plants elicited by the NADP metabolite nicotinic acid adenine dinucleotide phosphate. Proc Natl Acad Sci USA 97: 8693–8698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navazio L, Mariani P, Sanders D. (2001) Mobilization of Ca2+ by cyclic ADP-ribose from the endoplasmic reticulum of cauliflower florets. Plant Physiol 125: 2129–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BK, Cai X, Nebenführ A. (2007) A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J 51: 1126–1136 [DOI] [PubMed] [Google Scholar]
- Palmer AE, Jin C, Reed JC, Tsien RY. (2004) Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc Natl Acad Sci USA 101: 17404–17409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AE, Giacomello M, Kortemme T, Hires SA, Lev-Ram V, Baker D, Tsien RY. (2006) Ca2+ indicators based on computationally redesigned calmodulin-peptide pairs. Chem Biol 13: 521–530 [DOI] [PubMed] [Google Scholar]
- Palmer AE, Tsien RY. (2006) Measuring calcium signaling using genetically targetable fluorescent indicators. Nat Protoc 1: 1057–1065 [DOI] [PubMed] [Google Scholar]
- Parekh AB, Putney JW., Jr (2005) Store-operated calcium channels. Physiol Rev 85: 757–810 [DOI] [PubMed] [Google Scholar]
- Peiter E. (2011) The plant vacuole: emitter and receiver of calcium signals. Cell Calcium 50: 120–128 [DOI] [PubMed] [Google Scholar]
- Persson S, Harper J (2006) The ER and cell calcium. In DG Robinson, ed, The Plant Endoplasmic Reticulum: Plant Cell Monographs. Springer-Verlag, Heidelberg, pp 251–278 [Google Scholar]
- Pittman JK, Hirschi KD. (2003) Don’t shoot the (second) messenger: endomembrane transporters and binding proteins modulate cytosolic Ca2+ levels. Curr Opin Plant Biol 6: 257–262 [DOI] [PubMed] [Google Scholar]
- Qi Z, Stephens NR, Spalding EP. (2006) Calcium entry mediated by GLR3.3, an Arabidopsis glutamate receptor with a broad agonist profile. Plant Physiol 142: 963–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravier MA, Daro D, Roma LP, Jonas JC, Cheng-Xue R, Schuit FC, Gilon P. (2011) Mechanisms of control of the free Ca2+ concentration in the endoplasmic reticulum of mouse pancreatic β-cells: interplay with cell metabolism and [Ca2+]c and role of SERCA2b and SERCA3. Diabetes 60: 2533–2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincón-Zachary M, Teaster ND, Sparks JA, Valster AH, Motes CM, Blancaflor EB. (2010) Fluorescence resonance energy transfer-sensitized emission of yellow cameleon 3.60 reveals root zone-specific calcium signatures in Arabidopsis in response to aluminum and other trivalent cations. Plant Physiol 152: 1442–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Pozzan T. (2006) Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev 86: 369–408 [DOI] [PubMed] [Google Scholar]
- Sanders D, Pelloux J, Brownlee C, Harper JF. (2002) Calcium at the crossroads of signaling. Plant Cell (Suppl) 14: S401–S417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieberer BJ, Chabaud M, Timmers AC, Monin A, Fournier J, Barker DG. (2009) A nuclear-targeted cameleon demonstrates intranuclear Ca2+ spiking in Medicago truncatula root hairs in response to rhizobial nodulation factors. Plant Physiol 151: 1197–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding EP, Harper JF. (2011) The ins and outs of cellular Ca2+ transport. Curr Opin Plant Biol 14: 715–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stael S, Wurzinger B, Mair A, Mehlmer N, Vothknecht UC, Teige M. (2012) Plant organellar calcium signalling: an emerging field. J Exp Bot 63: 1525–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H, Liang F, Hwang I, Curran AC, Harper JF. (2000) Diversity and regulation of plant Ca2+ pumps: insights from expression in yeast. Annu Rev Plant Physiol Plant Mol Biol 51: 433–462 [DOI] [PubMed] [Google Scholar]
- Tanaka K, Swanson SJ, Gilroy S, Stacey G. (2010) Extracellular nucleotides elicit cytosolic free calcium oscillations in Arabidopsis. Plant Physiol 154: 705–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincill ED, Bieck AM, Spalding EP. (2012) Ca2+ conduction by an amino acid-gated ion channel related to glutamate receptors. Plant Physiol 159: 40–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincill ED, Clarin AE, Molenda JN, Spalding EP. (2013) Interacting glutamate receptor-like proteins in Phloem regulate lateral root initiation in Arabidopsis. Plant Cell 25: 1304–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waadt R, Kudla J. (2008) In planta visualization of protein interactions using bimolecular fluorescence complementation (BiFC). Cold Spring Harb Protoc 2008: pdb.prot4995, [DOI] [PubMed] [Google Scholar]
- Webb AAR, McAinsh MR, Taylor JE, Hetherington AM. (1996) Calcium ions as intracellular second messengers in higher plants. Adv Bot Res 22: 45–96 [Google Scholar]
- Weinl S, Held K, Schlücking K, Steinhorst L, Kuhlgert S, Hippler M, Kudla J. (2008) A plastid protein crucial for Ca2+-regulated stomatal responses. New Phytol 179: 675–686 [DOI] [PubMed] [Google Scholar]
- Wheeler GL, Brownlee C. (2008) Ca2+ signalling in plants and green algae: changing channels. Trends Plant Sci 13: 506–514 [DOI] [PubMed] [Google Scholar]
- Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ. (2007) An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2: e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Liang F, Hong B, Young JC, Sussman MR, Harper JF, Sze H. (2002) An endoplasmic reticulum-bound Ca2+/Mn2+ pump, ECA1, supports plant growth and confers tolerance to Mn2+ stress. Plant Physiol 130: 128–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Costa A, Leonhardt N, Siegel RS, Schroeder JI. (2008) Isolation of a strong Arabidopsis guard cell promoter and its potential as a research tool. Plant Methods 4: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JJ, Mehta S, Israelsson M, Godoski J, Grill E, Schroeder JI. (2006) CO2 signaling in guard cells: calcium sensitivity response modulation, a Ca2+-independent phase, and CO2 insensitivity of the gca2 mutant. Proc Natl Acad Sci USA 103: 7506–7511 [DOI] [PMC free article] [PubMed] [Google Scholar]