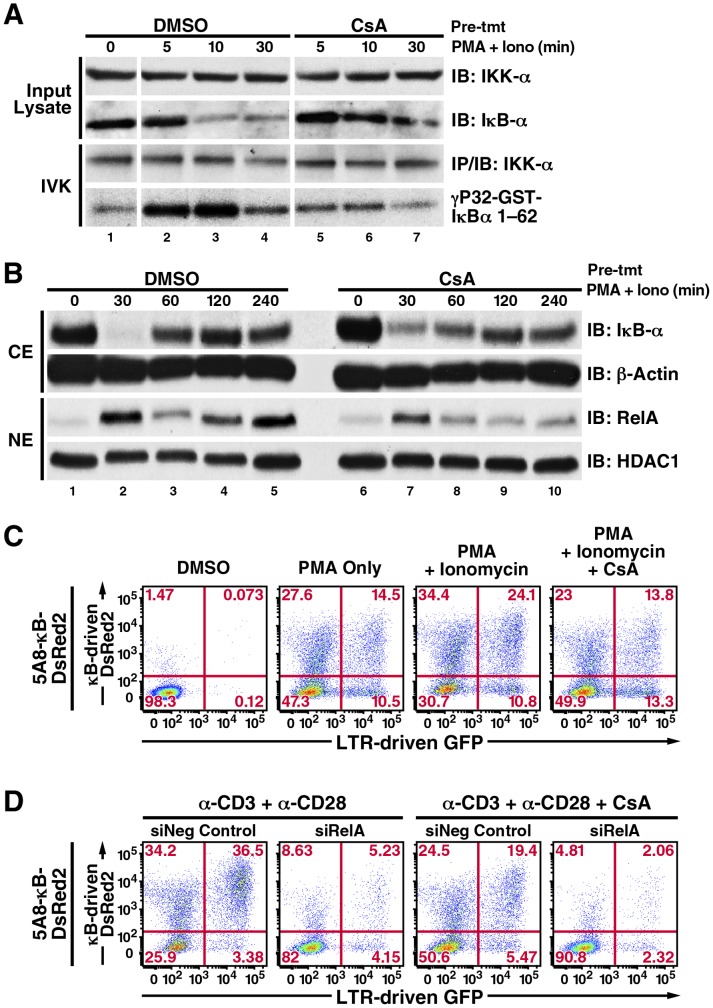
Figure 3. CsA reduces PKC-induced NF-κB/RelA activation and LTR-transcription in 5A8 cells.
A, in vitro kinase assay of IκBα phosphorylation by IKK. 5A8 cells were pretreated with DMSO or 500 nM CsA for 2 h, stimulated with 20 nM PMA and 2 µM ionomycin for the indicated times, lysed, and in vitro kinase assays was performed using glutathione-S-transferase IκBα (1–62) as the substrate as described [10]. CsA delayed the degradation of endogenous IκBα induced by PMA/ionomycin, which correlated with reduced phosphorylation of GST-IκBα by immunoprecipitated IKK-α. B, analysis of PMA/ionomycin-induced IκBα degradation and RelA nuclear translocation. 5A8 cells were treated with DMSO or 500 nM CsA for 2 h, stimulated with 20 nM PMA and 2 µM ionomycin, and fractionated into nuclear and cytoplasmic extracts. Immunoblotting analyses revealed that CsA interfered with complete degradation of cytoplasmic IκBα at 30 min and reduced its reappearance at 60 min. CsA also reduced the first and second rounds of nuclear NF-κB/RelA expression at 30 min and 120 min. C, characterization of 5A8-κB-DsRed2 cells. Cells were treated as in Figure 2C. Unlike 5A8-NFAT-DsRed2 cells, PMA alone induced both κB-dependent DsRed2 and LTR-driven GFP reporter activities (panel 2). Combined PMA/ionomycin stimulation further enhanced the activities of both reporters (panel 3), which were partially suppressed by CsA to levels similar to those after stimulation with PMA only (panel 4). D, effects of RelA knockdown on expression of κB-dependent DsRed2 and LTR-driven GFP. 5A8-κB-DsRed2 cells were nucleofected with negative control siRNA (panels 1 and 3) or siRNA against RelA (panels 2 and 4) twice within 48 h, followed by drug and antibody treatments as in Figure 2d. RelA knockdown suppressed both κB-DsRed2 and LTR-driven GFP reporter activities (panels 2 and 4).