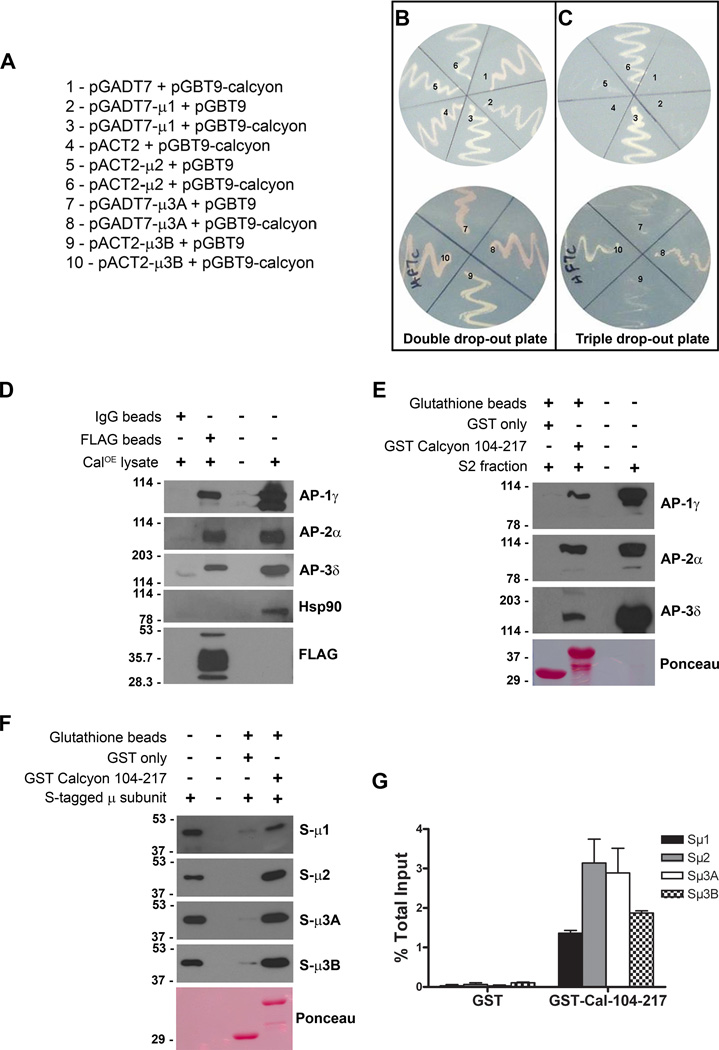
Figure 1. Calcyon directly interacts with ‘µ’ subunits of adaptor proteins.
A. Y2H bait and prey plasmid pairs tested. Plasmid pairs were transformed into HF7C cells, and plated in sectors labeled ‘one’ to ‘ten’ on the double (−Leu, −Trp, +His) and triple (−Leu, −Trp, −His) dropout plates shown in B and C, respectively. Growth of colonies co-transfected with pGBT9 calcyon (104–217) and pGADT7-µ1, pACT2-µ2, pGADT7-µ3A or pACT2-µ3B suggests prototrophy on histidine deficient media depends on AP interaction with calcyon. D. Immunoblots of proteins eluted following incubation of CalOE brain extracts with anti-FLAG or non-immune IgG beads as indicated by the plus and minus signs. Blots were probed with antibodies to γ, α, and δ subunits of AP-1, AP-2, and AP-3, respectively as well as with Hsp90 and FLAG antibodies. E. Immunoblots of proteins eluted after incubation of control mouse brain S2 fractions with GST or GST-calcyon 104–217 bound to glutathione resin. Blots were probed with antibodies to the γ, α, and δ AP subunits as in D. F. S-HRP detection of AP µ subunits eluted following incubation of resin bound GST or GST-calcyon 104–217 with purified S-tagged µ1, µ2, µ3A and µ3B subunits as indicated to the right of each panel. Ponceau S staining of the lower molecular weight region of the blots in E and F confirms that equivalent amounts of GST and GST-Calcyon 104–217 were used. G. Histogram showing the mean AP µ subunit binding to GST and GST-Calcyon 104–217 detected in three independent experiments expressed as a fraction of input. Error bars indicate the standard error of the mean (SEM).