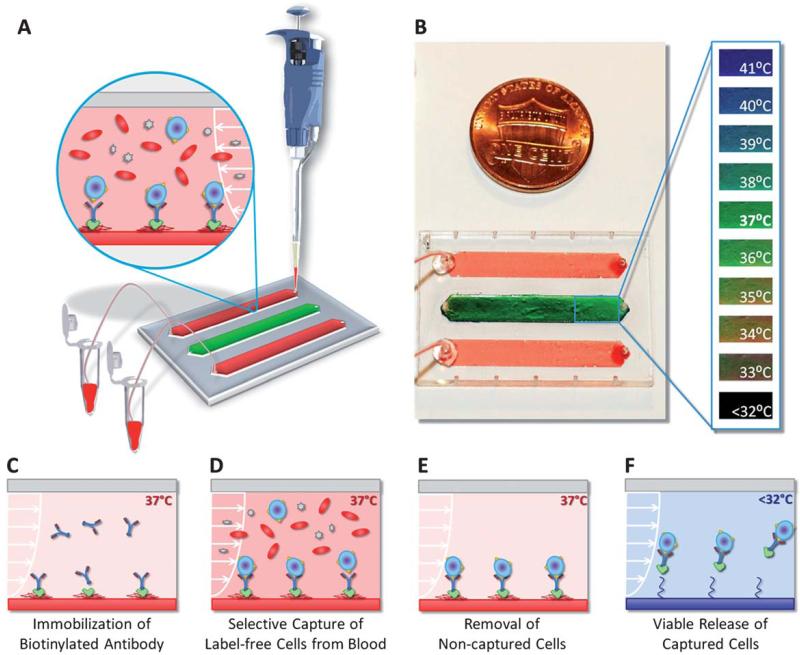
Fig. 1.
Thermoresponsive microfluidic chip developed for releasing selectively captured cells from blood. (A) The microfluidic chip was composed of three parallel channels (4 mm × 22 mm × 80 μm), one of which (middle channel) was used as the temperature indicator channel. Blood was introduced into the top and bottom release channels with a manual pipette. The tubing connected to the outlet ports allowed the collection of the released cells in microcentrifuge tubes. (B) The middle channel was coated with temperature sensitive liquid crystal dye, which was responsive between 35 °C (red-orange) and 40 °C (blue-purple). At the target temperature of 37 °C, the middle channel displayed green color. (C) Schematic drawing of the working principle of label-free selective capture from whole blood and controlled release of cells in thermoresponsive microfluidic channels. Biotin binding protein (Neutravidin) and biotinylated antibody (Anti-CD4 or Anti-CD34) were immobilized on the PNIPAAm channel surface at 37 °C. (D) Pre-warmed blood sample (at 37 °C) was injected into the microfluidic channel, and the CD4+ cells or the CD34+ cells in blood were captured on the channel surface. (E) The non-captured cells in the channels were rinsed off and the red blood cells were lysed. (F) The microchip was then cooled down below 32 °C (in less than 5 minutes). The released cells were rinsed out of the channels and collected at the channel outlet.