Figure 2. The K-rich domain of STIM2 forms an α-helix and binds PI(4,5)P2 as dimer.
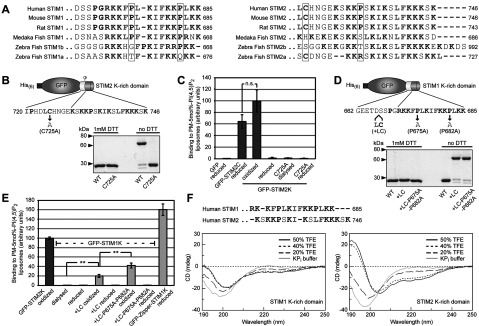
(A) Alignment of extreme C-termini of STIM proteins from different species. Alignment of K-rich domains from STIM1 in mammals and fishes are shown on the left and STIM2 on the right. Conservation of proline and cysteine residues are highlighted (B) Cartoon of GFP fused to the last 27 residues of STIM2 (GFP–STIM2K) where Cys725 is depicted as grey circle. Coomassie Brilliant Blue stained GFP–STIM2K [WT (wild-type)] and GFP–STIM2K with a C725A mutation (C725A) after overnight dialysis in HK buffer (25 mM Hepes/KOH pH 7.5, 150 mM NaCl) and separation by SDS/PAGE under reducing and non-reducing conditions. (C) Binding of 1 μM reduced GFP and GFP–STIM2C, oxidized and reduced GFP–STIM2K, GFP–STIM2K–C725A dialysed against DTT-free buffer and reduced GFP–STIM2K–C725A to PM-like liposomes with 5 mol% PI(4,5)P2. Binding of oxidized GFP–STIM2K was set to 100. Bars indicate mean±S.D. from at least three experiments and n.s. (not significant) according to student's t test. (D) Cartoon of GFP fused to the last 24 residues of STIM1 (GFP–STIM1K) and positions of the introduced Leu and Cys residues and Pro to Ala mutations. Coomassie Brilliant Blue stained GFP–STIM1K (WT), GFP–STIM1K+LC and GFP–STIM1K+LC–P675A–P682A after overnight dialysis in HK buffer with and without 1 mM DTT and separation by SDS/PAGE under reducing and non-reducing conditions. (E) Binding of 1 μM oxidized GFP–STIM2K, GFP–STIM1K dialysed against DTT-free buffer, reduced GFP–STIM1K, oxidized and reduced GFP–STIM1K+LC, GFP–STIM1K+LC-P675A-P682A and reduced GFP-Zipper-STIM1K to PM-like liposomes with 5 mol% PI(4,5)P2. Binding of 1 μM oxidized GFP–STIM2K was set to 100. Bars indicate means±S.D. from at least three experiments (**P<0.005 according to student's t test). (F) Sequence alignment of peptides of human STIM1 (residues 671–685) and STIM2 (residues 730–746) corresponding to the K-rich domains used for CD. CD spectra of 150 μM peptide in 5 mM KPi buffer pH 7.6 containing 0, 20, 40 and 50% TFE, is shown below the alignment.
