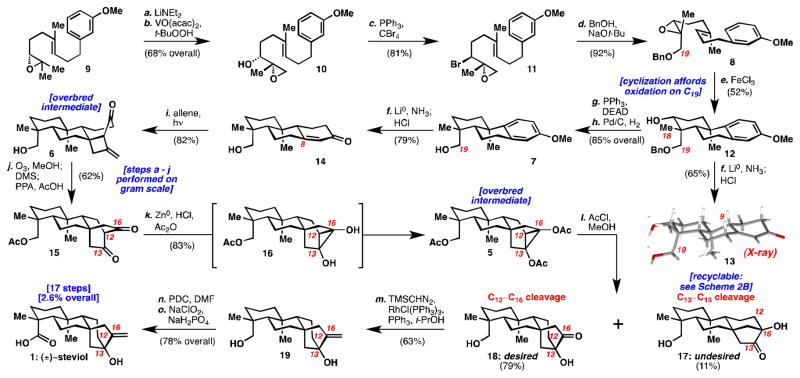
Scheme 1. Total synthesis of (±)-steviol (1).
Reagents and Conditions: (a) LiNEt2 (3 equiv), THF, 60 °C, 2 h (92%); (b) VO(acac)2 (0.25 equiv), t-BuOOH (5 M in decane) (1.6 equiv), benzene, 6 °C, 2 h (74% or 83% BRSM); (c) CBr4 (3 equiv), PPh3 (2.9 equiv), i-Pr2NEt (3.3 equiv), CH2Cl2, −10 °C, 12 h (81%); (d) NaOt-Bu (2.4 equiv) benzyl alcohol (solvent), 100 °C, 3 h (92%); (e) FeCl3 (2 equiv), CH2Cl2, rt, 3 h (52%); (f) Li0 (50 equiv), NH3, THF; t-BuOH, −78 °C to −45 °C, 2 h; 4M HCl in dioxane, rt, 30 min (79%); (g) DEAD (5 equiv), PPh3 (5 equiv), THF, 70 °C, 5 h (91%); (h) H2, Pd/C (10 wt%)(10 mol%), EtOAc, rt, 7 h (93%); (i) allene, CH2Cl2, rt, 450 W Hg-lamp, pyrex, 12 h (82%); (j) O3, MeOH, −78 °C, 5 min; Me2S, rt, 30 min; AcOH: PPA (9:1), 110 °C, 12 h (62%); (k) HCl(g), Ac2O (solvent), act. Zn0 (60 equiv), 0 °C, 45 min; (l) AcCl (3 M in MeOH), 0–6 °C, 12 h (79% 18 and 11% 17); (m) PPh3 (6.6 equiv), RhCl(PPh3)3 (5 mol%), THF, i-PrOH; TMSCHN2 (20 equiv), 48 h (63%); (n) PDC (5 equiv), DMF, rt, 18 h, (92%); (o) NaClO2 (6 equiv), NaH2PO4(10 equiv), 2-methyl-2-butene (10 equiv), THF/t-BuOH, 0 °C to rt, 16 h (85%). (DEAD = diethyl azodicarboxylate, PPA = polyphosphoric acid, PDC = pyridinium dichromate, DMF = N,N-dimethylformamide).