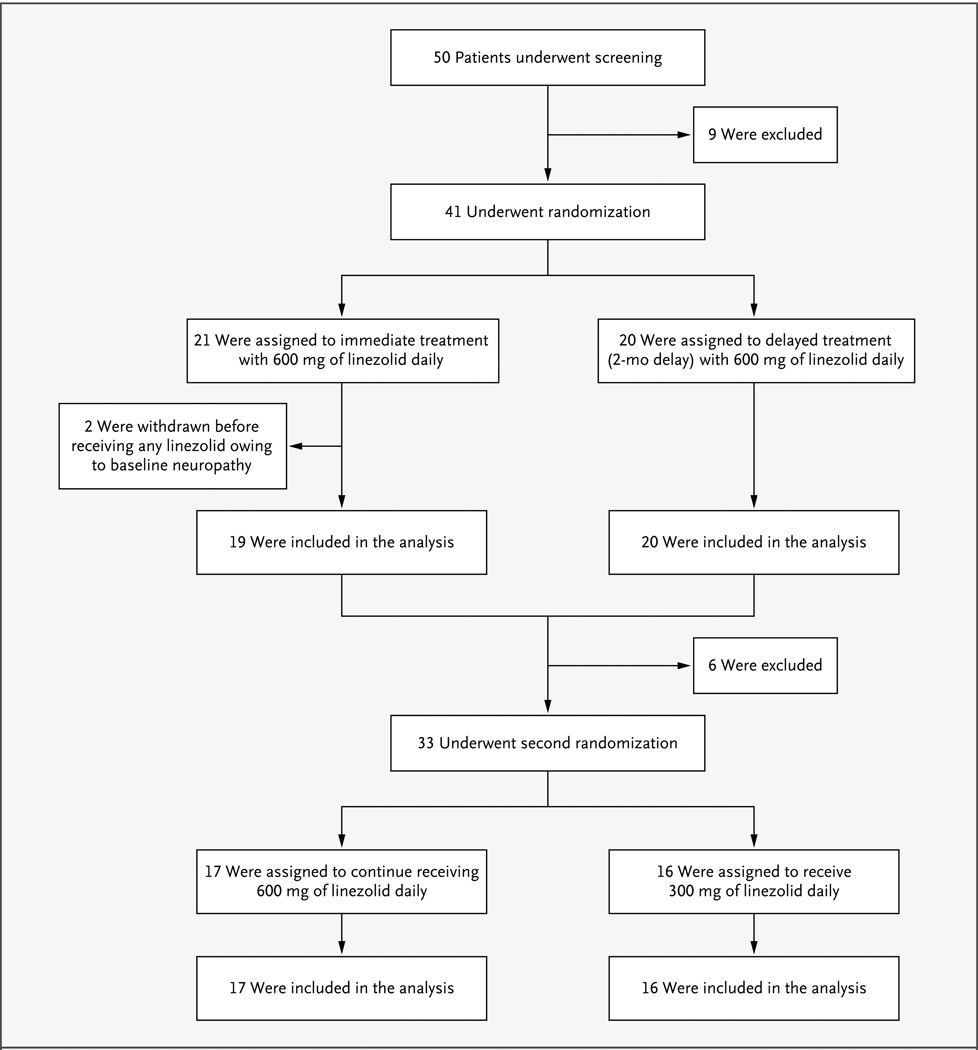
Figure 1. Enrollment, Randomization, and Follow-Up of the Study Patients.
Between December 2008 and May 2011, a total of 50 patients were screened for eligibility and 41 underwent randomization. Two patients were subsequently withdrawn owing to preexisting peripheral neuropathy that was discovered during the baseline examination; the remaining 39 patients were included in the modified intention-to-treat analysis. Two other patients who withdrew before culture conversion were considered to have treatment failure: 1 patient, who had an adverse event requiring a drug holiday, was withdrawn 79 days after starting treatment with linezolid because the drug holiday exceeded the protocol-specified window (28 days before sputum-culture conversion and 42 days after sputum-culture conversion); the other patient was withdrawn 32 days after study entry because of a diagnosis of advanced colon cancer (this patient was in the delayed-start group and had not received any linezolid). Thirty-three patients underwent the second randomization; 17 patients were randomly assigned to continue receiving linezolid at a dose of 600 mg per day, and 16 to receive the reduced dose of 300 mg per day. The 6 patients who did not undergo the second randomization included 4 who had dose reductions due to adverse events before culture conversion and the 2 withdrawn patients mentioned above who were included in the modified intention-to-treat analysis as having treatment failure.