Figure 4.
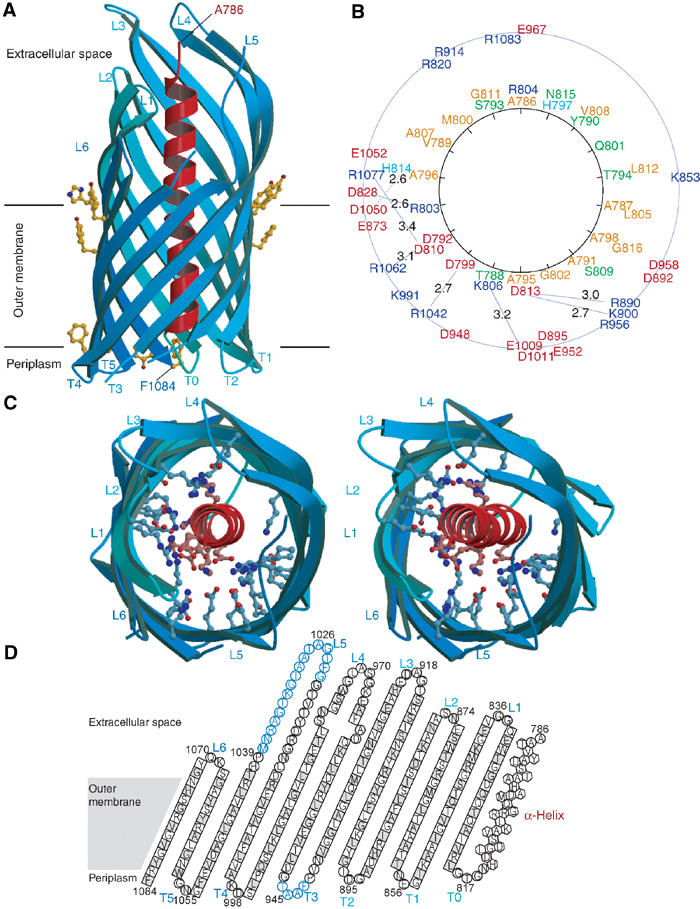
Crystal structure of NalPβ. (A) Side view of NalPβ in space group P6122 shows a 12-stranded β-barrel (blue ribbon representation) with a shear number of 14. The hydrophobic membrane-embedded region is flanked by aromatic residues (yellow ball-and-stick). The periplasmic side is characterised by short turns (T0–T5) and the extracellular side by longer loops (L1–L6) connecting the alternating β-strands. An α-helical ‘plug' (red ribbon representation) is connected to the barrel via T0 and positions the N-terminus of the translocator domain (Ala 786) at the extracellular side. (B) Schematic representation of the mixed character of the α-helix and its interactions with the β-barrel wall with the α-helical residues depicted on a helical wheel. Colour coding: positively charged residues in blue, negatively charged residues in red, hydrophilic residues in green and nonpolar residues in orange. The distances between charged groups of the helix and the barrel wall are indicated in Å. (C) Stereo top view of the NalPβ β-barrel in the same orientation as in (B). The barrel interior is highly hydrophilic due to the presence of many charged amino acids (ball-and-stick representation). (D) Topology model of NalPβ; residues pointing outwards from the β-barrel are indicated in grey. Amino acids in β-strands are indicated as squares, in α-helix as hexagons and in loops as circles. Amino acids not visible in the electron density of space group P6122 are indicated in blue.
