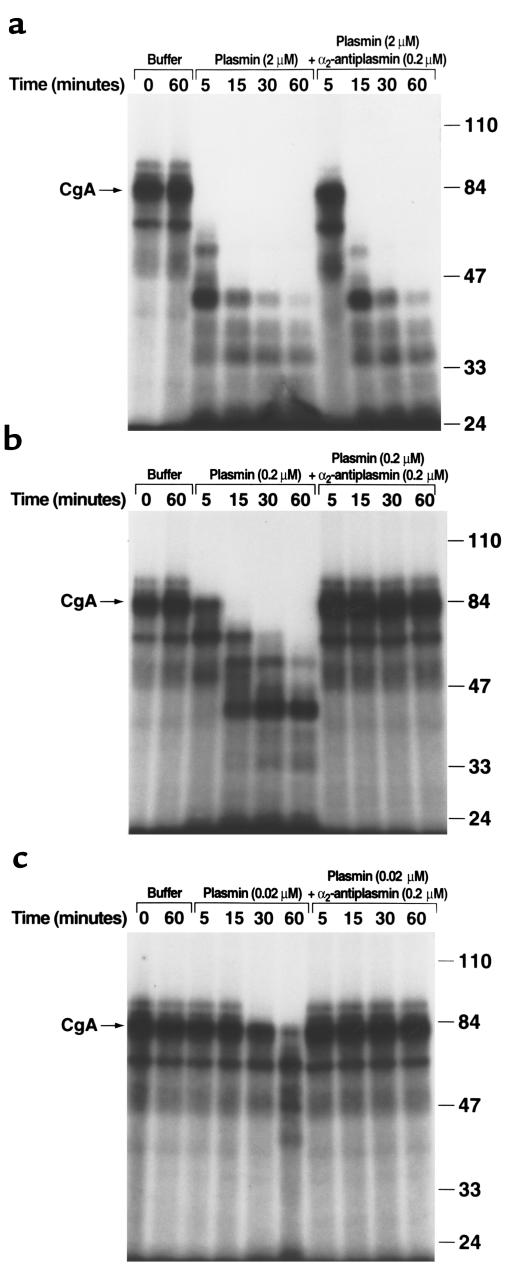
Figure 1.
Cleavage of CgA by plasmin. Human 125I-CgA (1.5 nM) was incubated with either buffer (PBS) or streptokinase (100 U/ml) plus 2 μM plasminogen (a), 0.2 μM plasminogen (b), or 0.02 μM plasminogen (c) in 0.01 M Tris Cl, pH 8.0, 0.15 M NaCl, at 37°C for the indicated times in the presence or absence of 0.2 μM α2-antiplasmin. The generation of active plasmin was verified spectrophotometrically as cleavage of the chromogenic substrate, S-2251, D-Val-Leu-Lys-paranitroanilide. Samples were electrophoresed on 8% SDS-PAGE under nonreducing conditions. Mr size standards (in thousands) are indicated on the right. In controls, neither plasminogen alone nor streptokinase alone cleaved the parent CgA molecule (data not shown).