Figure 11.
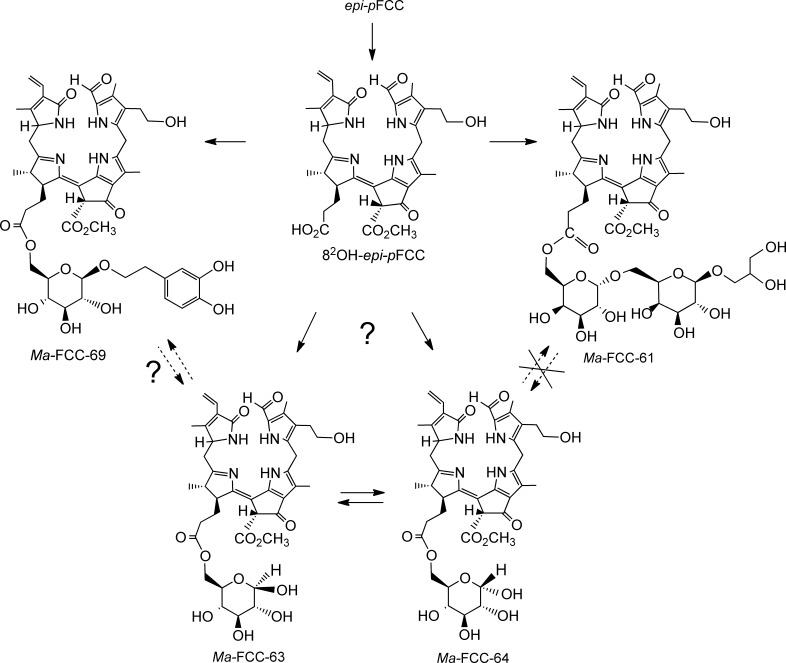
Structural outline of the proposed later part of chlorophyll degradation in senescent leaves of bananas, beginning with epi-pFCC, the C1 epimer of the primary FCC (pFCC).[2, 9, 13] Hydroxylation at the ethyl side chain of ring B leads to the secondary FCC (82-OH-epi-pFCC), which is proposed to be the common precursor of all downstream catabolites in banana leaves. Modifications forming complex propionate esters stop the common path and lead to hypermodified fluorescent chlorophyll catabolites (hmFCCs), such as Ma-FCC-61 and Ma-FCC-69. Possible cleavage at the anomeric center of the glucopyranosyl moiety of Ma-FCC-69 could give monoglycosylated Ma-FCC-63, which the other way around could be a potential precursor of Ma-FCC-69. As shown, isomerization of Ma-FCC-63 to its anomer Ma-FCC-64 and vice versa occurs in protic solutions.
