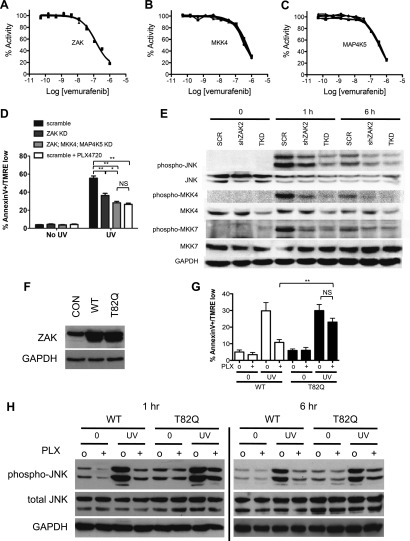
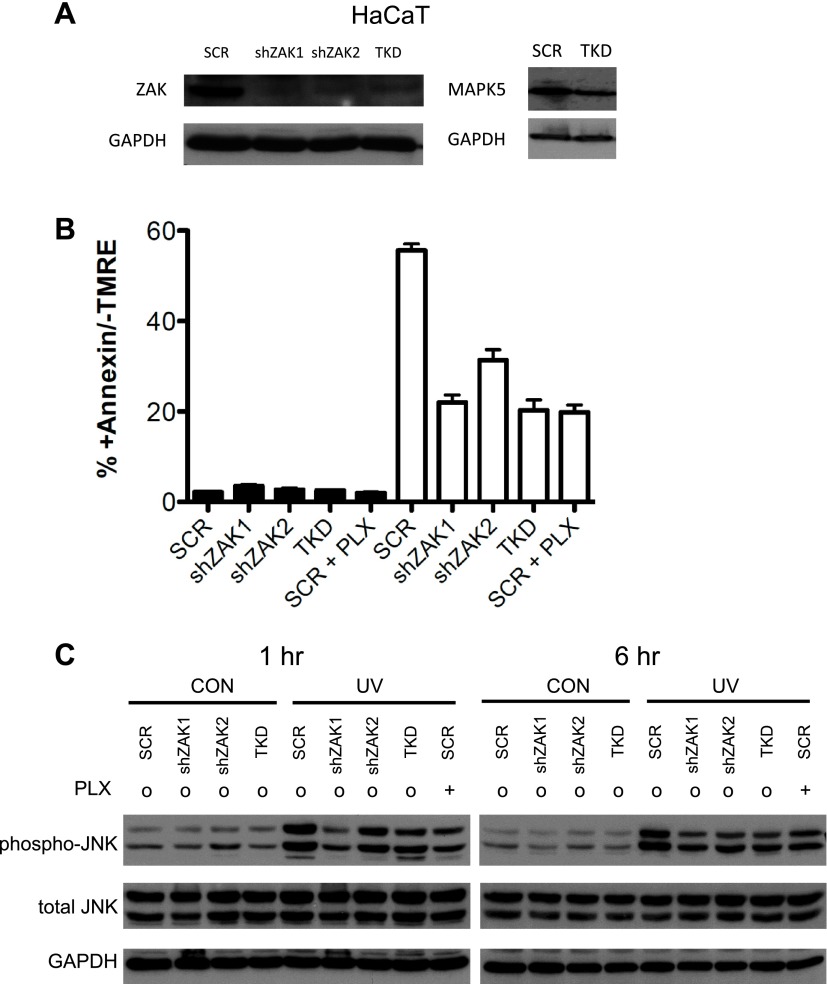
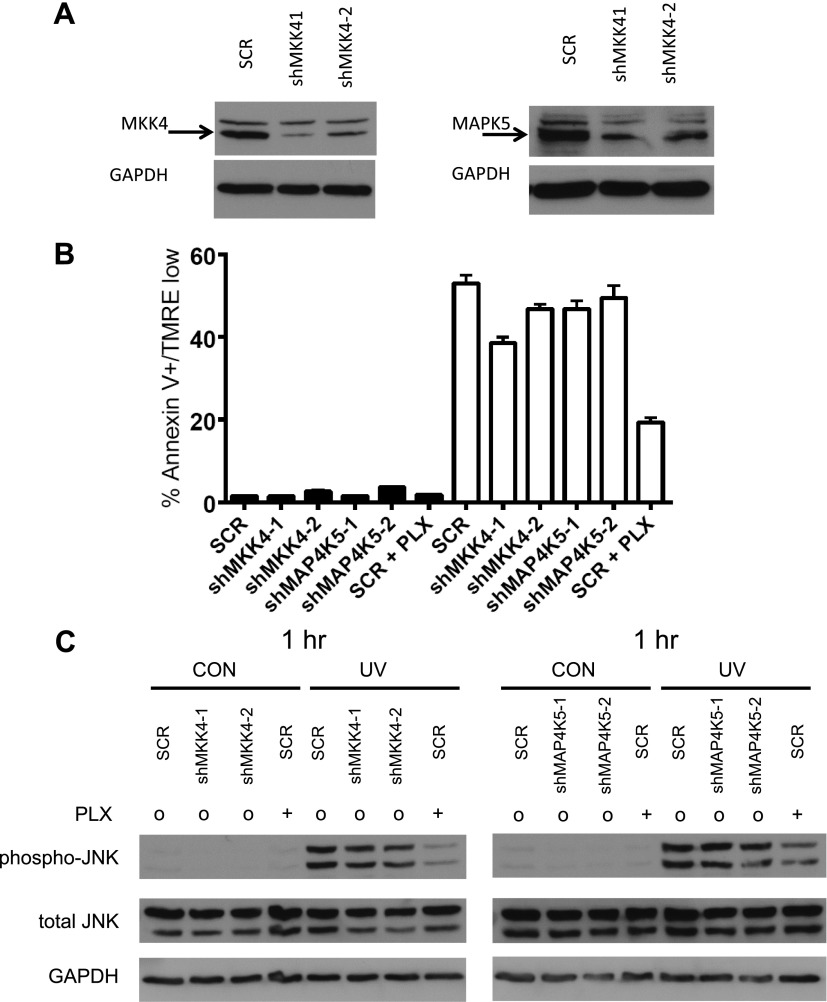
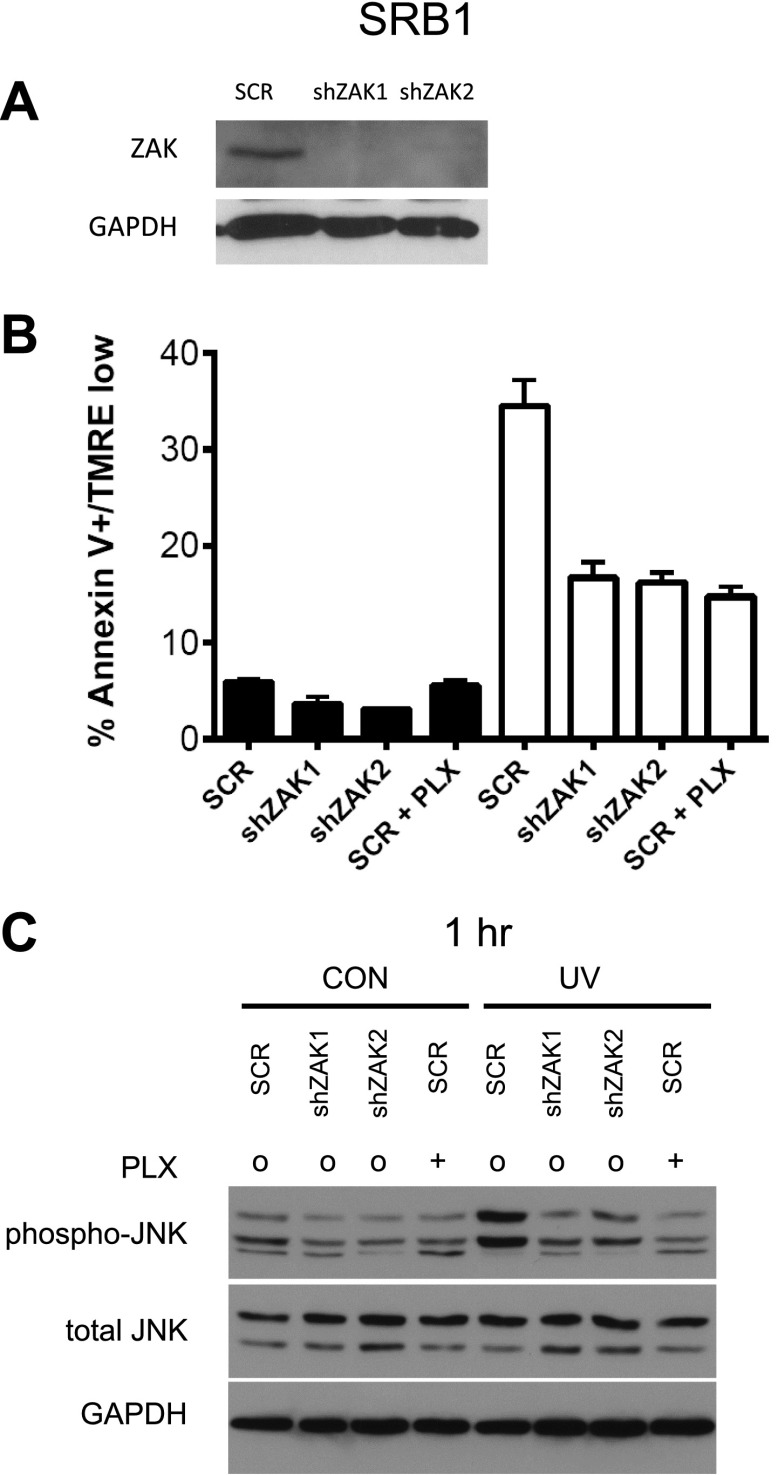
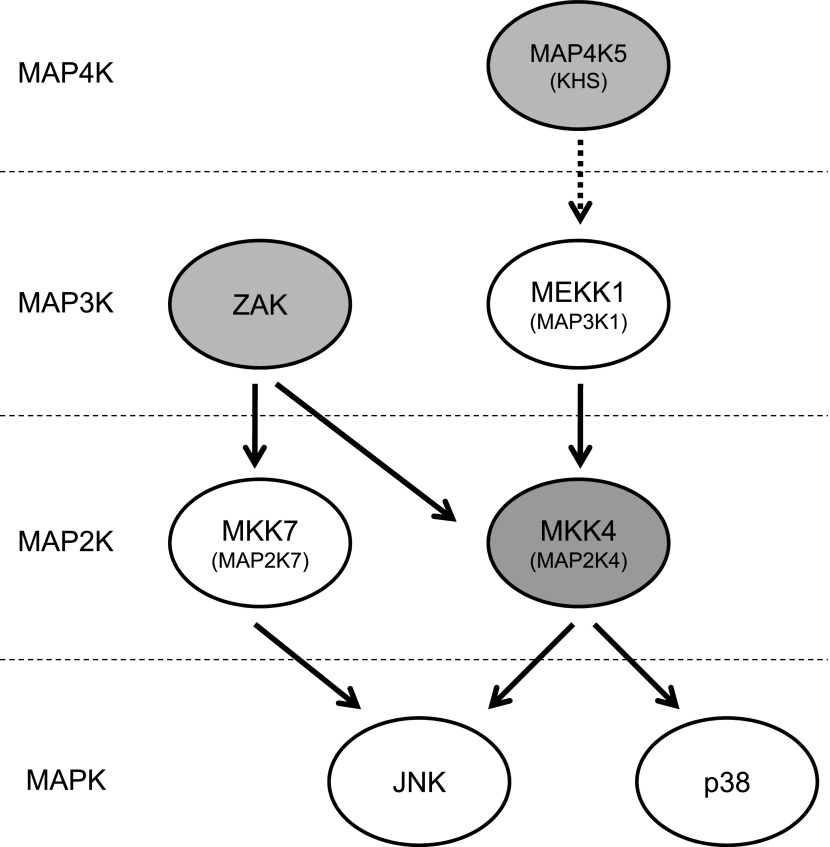
Figure 3. PLX4720 and vemurafenib suppress apoptosis and JNK signaling through inhibition of off-target kinases.
(A–C) In-vitro kinase assays for ZAK, MKK4, and MAP4K5 were performed across a 10-point concentration range from 0.05 to 1000 nM in triplicate, revealing significant inhibition of kinase activity within the nM range for vemurafenib. (D) Lentiviral shRNA knockdown of ZAK singly or in combination with MKK4 and MAP4K5 (triple knockdown, ‘TKD’) was performed revealing potent suppression of apoptosis as measured by FACS for Annexin V+, TMRE-low cells (n = 5, ‘*’ denotes statistical significance at p<0.05, ‘**’ at p<0.01, ‘NS’ is not significant) at 24 hr following single dose UVB irradiation at 720 J/m2. ZAK knockdown and triple knockdown cells exhibit 70% and 94% suppression of apoptosis, respectively, relative to PLX4720-treated cells expressing a non-suppressing shRNA control (scramble, ‘SCR’). (E) Western blots of lysates obtained at 1 and 6 hr post-UV irradiation show potent induction of phospho-MKK4, phospho-MKK7, and phospho-JNK which are all suppressed with progressively increasing effect in ZAK single knockdown (‘shZAK2’) and triple knockdown (‘TKD’) HaCaT cells. (F) Western blots of HaCaT cells electroporated with pcDNA3-wild-type (WT) ZAK and the gatekeeper mutant pcDNA3-(T82Q) ZAK show equivalent expression. (G) HaCaT cells overexpressing ZAK (WT) and ZAK (T82Q) were irradiated with a single dose of UVB irradiation at 720 J/m2 in the absence (‘o’) and presence (‘+’) of 1 μM PLX4720 and apoptosis measured by FACS for Annexin V+, TMRE-low cells (n = 4, ‘**’ at p<0.01, ‘NS’ is not significant) at 24 hr. ZAK (WT) cells are sensitive to PLX4720-mediated suppression of apoptosis (bar 3 vs 4), but drug-treated ZAK (T82Q)-expressing cells undergo significantly more apoptosis than drug-treated ZAK (WT) cells (bar 4 vs 8), with bypass of PLX4720-induced suppression as compared to drug-treated ZAK (WT) cells (paired t-test, p=0.005). (H) Western blots of ZAK (WT) and ZAK (T82Q)-expressing HaCaT cells at 1 hr and 6 hr post-irradiation show that phospho-JNK activation is intact in both cell lines in the absence of drug (lanes 3, 7), but that drug-treated ZAK (T82Q)-expressing HaCaT cells have significantly more phospho-JNK activation at both 1 and 6 hr post-irradiation, as compared to drug-treated ZAK (WT)-expressing cells (lane 4 vs 8).