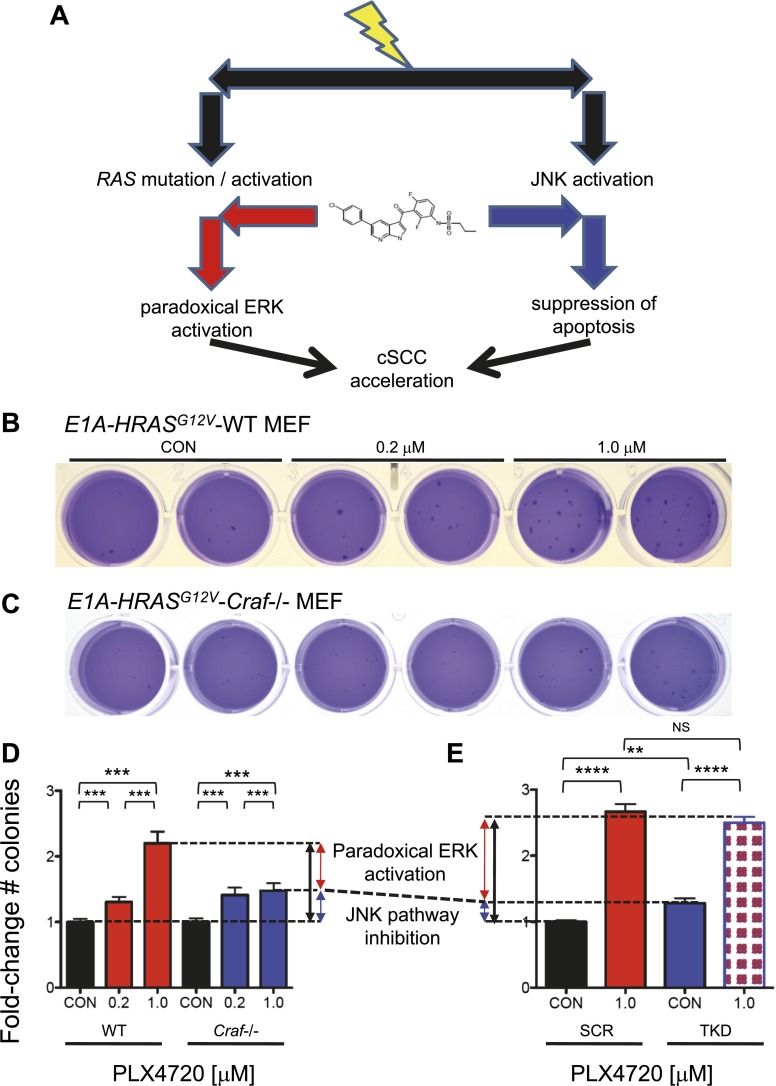
Figure 6. Paradoxical ERK activation and JNK pathway inhibition make significant and separable contributions to BRAFi-induced growth.
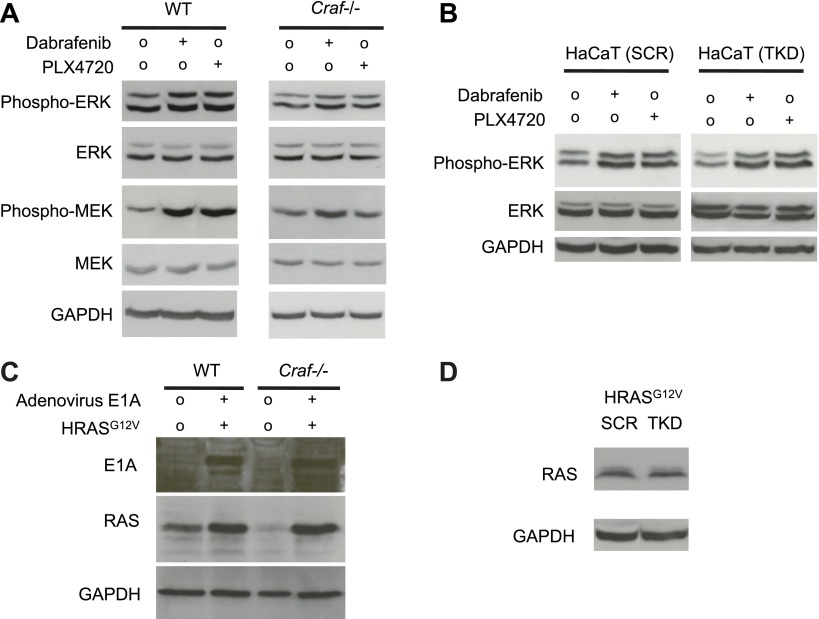
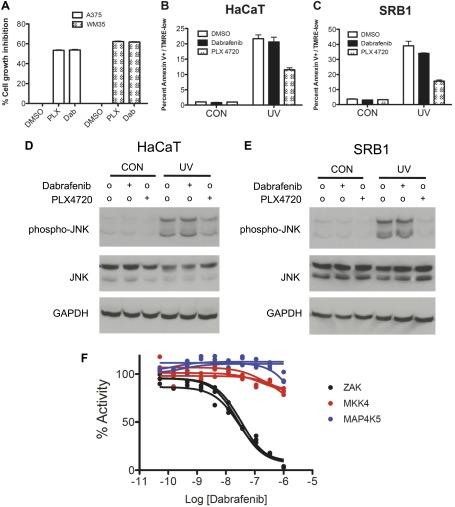
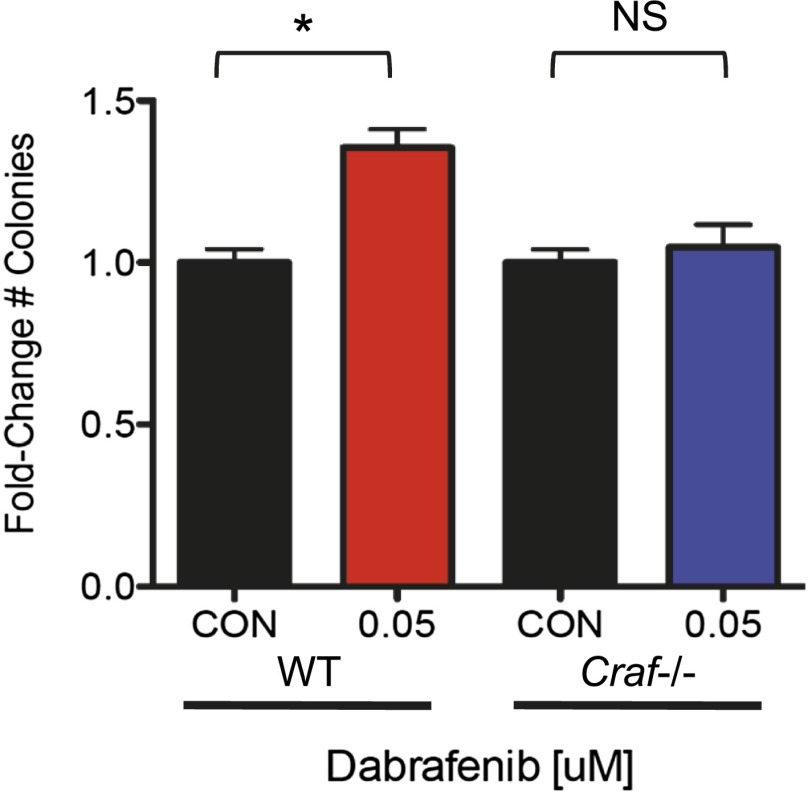
(A) We envision two separable, parallel mechanisms by which PLX4720 and vemurafenib contribute to cSCC development. Drug-induced paradoxical ERK activation and inhibition of JNK signaling occur in parallel, but the former depends on intact CRAF. (B and C) Representative soft agar colonies of E1A and HRASG12V-transformed wild-type (WT) (B) and Craf−/− (C) MEFs, following exposure to 0.2 μM and 1.0 μM PLX4720 over 4–6 weeks show significant colony-forming advantages conferred by BRAFi. (D) The fold-change in colony counts of transformed wild-type (WT) (n = 22 replicates) and Craf−/− (n = 14 replicates) MEFs demonstrate a dose-dependent increase in colonies, particularly for WT MEFs. The difference between colony formation advantages conferred by 1.0 μM PLX4720 in WT vs Craf−/− MEFs was interpreted to reflect the contribution of paradoxical ERK signaling (red arrow), which depends upon Craf, and is 60% of the total effect (black arrow), with the remainder composed of other effects including JNK inhibition (blue arrow). All differences between each MEF population were significant (‘***’, p<0.001) (E) The fold-change in colony counts of transformed HaCaT cells with (‘TKD’) and without (‘SCR’) triple lentiviral shRNA knockdown of ZAK, MAP4K5, and MAP2K4, show significant differences between 1.0 μM PLX4720-treated and control-treated conditions (‘****’, p<10−10). Importantly, untreated TKD cells had a significant advantage over untreated SCR HaCaT cells (‘**’, p<0.01), which we interpreted to be the contribution of JNK signaling inhibition, of 17.6% (blue arrow). Drug-treated SCR and TKD cells both had a similar degree of total colony formation advantage (averaged as black arrow), as expected, since the TKD cells are not expected to have any additional suppression of JNK signaling in the presence of drug (‘NS’, p=0.17, Figure 3D). Therefore, the colony counts for these two distinct systems (D and E), when taken together, show that JNK pathway inhibition accounts for approximately 17.6–40% and paradoxical ERK activation accounts for approximately 60–82.4% of the total effects of PLX4720 on tumor growth.