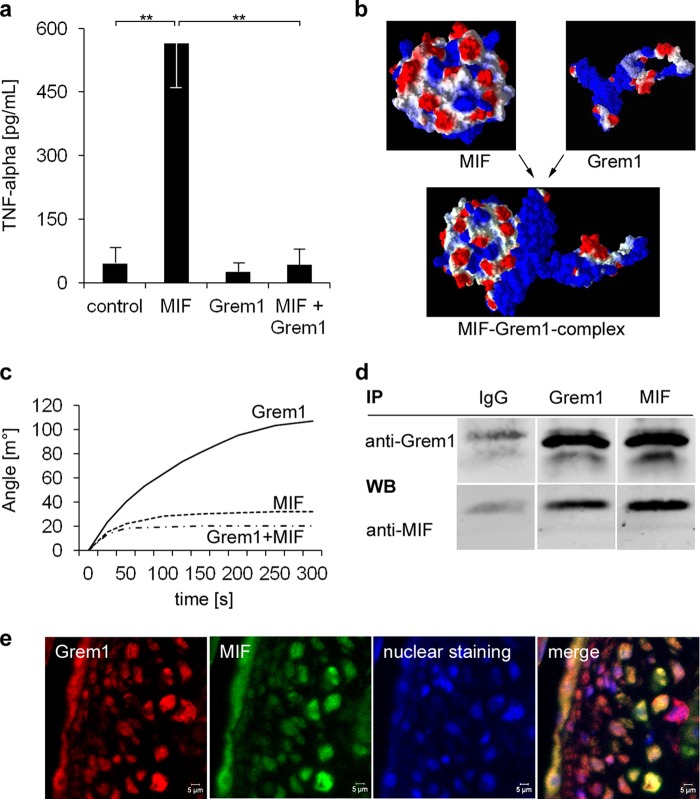
FIGURE 3.
Gremlin-1 inhibits MIF-induced secretion of TNF-α of monocytes and is a MIF-binding protein. a, macrophages were stimulated with MIF (0.25 μg/ml), Gremlin-1 (0.5 μg/ml), or a combination of both for 12 h. The concentration of TNF-α in the cell supernatant was determined by ELISA. Data represent the mean ± S.E. of three experiments. (**, indicates statistical significance of p < 0.01). b, computational modeling of the protein structures of MIF and Gremlin-1 and their predicted interaction with an estimated binding affinity of KD = 65 nm. Molecular modes were generated with Molegro Virtual Docker 5. The surface charge distribution is shown; negative charge is in red and positive charge in blue. c, surface plasmon resonance analysis. The binding of Gremlin-1 (100 nm), Gremlin-1 (100 nm) that has been preincubated with an excess of MIF (800 nm) for 10 min, and MIF alone (control) to the sensor surface coated with MIF (51–57) is shown. d, co-immunoprecipitation of the Gremlin-1/MIF complexes of monocyte cell extracts. Anti-Gremlin-1 immunoprecipitation (IP) followed by anti-MIF Western blotting (WB, lower panel) or anti-MIF immunoprecipitation followed by anti-gremlin-1 Western blotting (upper panel). Control: immunoprecipitation with an irrelevant idiotypic IgG. Representative results of five independently performed IP experiments are shown. e, co-localization of Gremlin-1 and MIF in aortic atherosclerotic lesions of ApoE−/− mice. Paraffin-embedded aortic tissue from 16-week-old ApoE−/− mice was stained with mAb directed against Gremlin-1 or MIF, respectively. Immunostainings were analyzed by confocal microscopy.