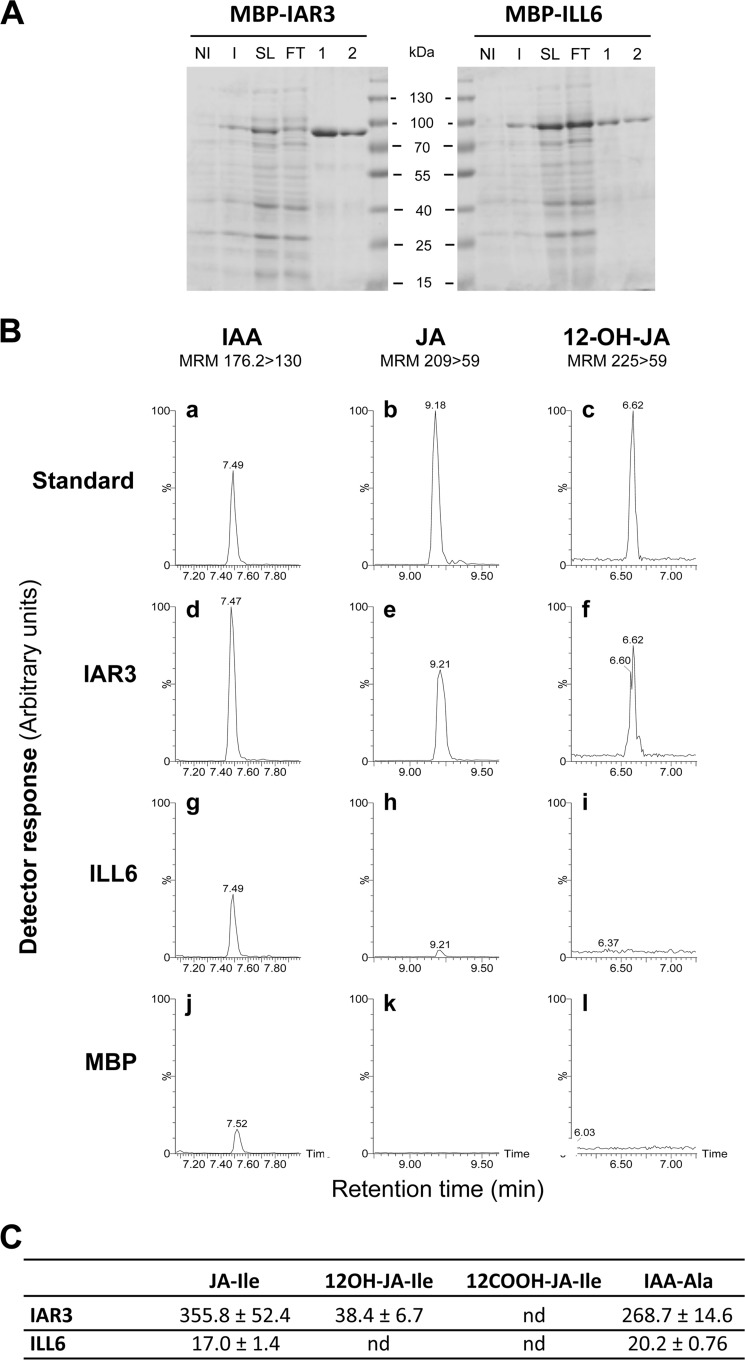
FIGURE 4.
Purification and enzymatic activity of recombinant IAR3 and ILL6 amidohydrolases. A, purification steps were analyzed by 12.5% SDS-PAGE. Total proteins from bacteria transformed with plasmids expressing fusion proteins were analyzed before (non-induced, NI) and after (induced, I) induction with 0.5 mm isopropyl 1-thio-β-d-galactopyranoside for 4 h. Fusion proteins are visible after induction. His-tagged proteins present in soluble lysate (SL) were purified by affinity chromatography. FT, flow through. 1 and 2, elution fractions 1 and 2. Molecular mass markers are indicated. Proteins were visualized by Coomassie staining. B, 20 μg of either protein were incubated with the following amino acid conjugates as candidate substrates: IAA-Ala, JA-Ile, and 12OH-JA-Ile. LC chromatograms are shown where the cleavage products IAA (panels a, d, g, and j), JA (panels b, e, h, and k), or 12OH-JA (panels c, f, i, and l) were separated by UPLC-MS/MS and detected by their indicated multiple reaction monitoring transitions. C, specific activities (pmol/h/mg of protein) were determined by incubation of purified enzymes for 90 min with 100 μm substrates and quantification of cleavage products with authentic standards curves. Values represent mean ± S.E. (n = 3). nd, not detected.