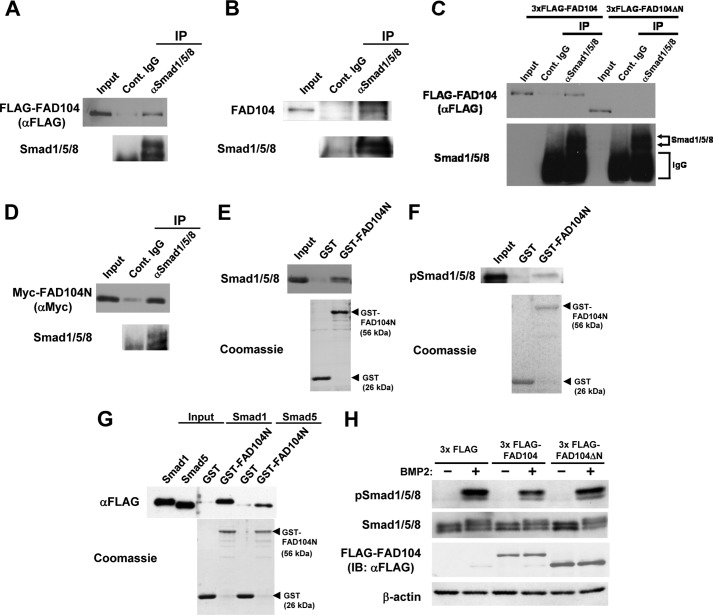
FIGURE 10.
FAD104 interacts with Smad1/5/8, and the N terminus of FAD104 is required for inhibition of Smad1/5/8 phosphorylation. A, the interactions of FAD104 with Smad1/5/8 by an IP assay. HeLa cells were transfected with 3xFLAG-FAD104 expression plasmids. IP experiments were performed using anti-Smad1/5/8 antibody. Normal rabbit IgG was used as a negative control. Immunoprecipitates and 0.5% input were resolved and detected by Western blotting with anti-FLAG antibody. B, interaction between FAD104 and Smad1/5/8 under physiological conditions. Lysates from intact C2C12 cells were immunoprecipitated using an antibody against Smad1/5/8. Immunoprecipitates and 0.5% input were resolved and detected by Western blotting with anti-FAD104 antibody. C, interaction of FAD104ΔN with Smad1/5/8 by IP assay. HeLa cells in a 10-cm culture dish were transfected with FLAG-FAD104 (15 μg) or FLAG-FAD104ΔN (1.875 μg) expression plasmids to equalize the amount of FLAG-FAD104 and FLAG-FAD104ΔN. IP experiments were performed by anti-Smad1/5/8 antibody. Normal rabbit IgG was used as a negative control. Immunoprecipitates and 0.2% input were resolved and detected by Western blotting with anti-FLAG antibody. D, interaction of the N terminus of FAD104 with Smad1/5/8 by IP assay. HeLa cells were transfected with Myc-FAD104N expression plasmids. IP experiments were performed by anti-Smad1/5/8 antibody. Normal rabbit IgG was used as a negative control. Immunoprecipitates and 0.2% input were resolved and detected by Western blotting with anti-Myc antibody. E, interaction of the N terminus of FAD104 with Smad1/5/8 via the GST pull-down assay. Cell lysates prepared from C2C12 cells were used for a GST pull-down assay with GST or GST-FAD104N. Bottom, Coomassie Blue staining of the GST proteins. Bound protein samples were immunoblotted with anti-Smad1/5/8 antibody. The input volume was 5% of that of the cell lysates for the pull-down assay. F, interaction of the N terminus of FAD104 with phospho-Smad1/5/8 via the GST pull-down assay. The cell lysates prepared from C2C12 cells treated BMP2 were used for a GST pull-down assay with GST or GST-FAD104N. Bottom, indicates Coomassie Blue staining of the GST proteins. Bound protein samples were immunoblotted with anti-phospho-Smad1/5/8 antibody. The input volume was 5% of that of the cell lysates for the pull-down assay. G, direct interaction between the N terminus of FAD104 and Smad1/5/8. FLAG-tagged Smad1 and Smad5 expressed in E. coli cells were purified using FLAG M2 affinity gel and eluted. The eluates were used for a GST pull-down assay with GST or GST-FAD104N. Bottom, Coomassie Blue staining of the GST proteins. Bound protein samples were immunoblotted with anti-FLAG antibody. The input volume was 5% of that of the eluates for the pull-down assay. H, Smad1/5/8 phosphorylation levels in C2C12 cells introduced 3xFLAG, 3xFLAG FAD104, or 3xFLAG FAD104ΔN expression plasmids. C2C12 cells transiently transfected with each plasmid were starved for 6 h and treated with BMP2 for 1 h. The Smad1/5/8 phosphorylation and protein levels of total Smad1/5/8 were determined by Western blotting. β-Actin expression was used as a control. A–H show typical results. Similar results were obtained in at least two independent experiments.