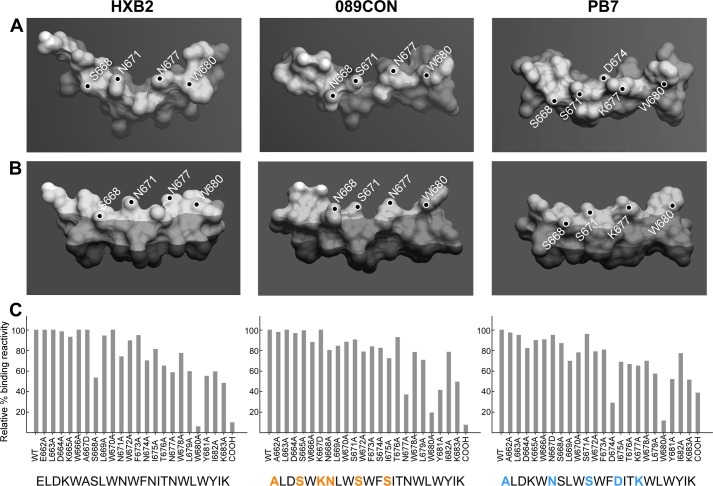
FIGURE 4.
Stereochemistry and immunogenicity of MPER segments from three different viral strains. A, top, and B, side views of HxB2 (left), 089CON (middle), and PB7 (right) MPER peptides shown as molecular surface representations embedded on viral membrane surfaces. Dark-shaded region indicates acyl chain region of the lipid bilayer, boundary between light- and medium-shaded region marks the position of phosphate in the lipid headgroup area, and the small, unshaded parts indicate complete solvent exposure. Key solvent-exposed residues are labeled. C, fine epitope specificities of MPER responses elicited by three different MPER/liposome vaccines. Single alanine-substituted peptides of each position of the various Npalm-MPER immunogens were tested for their reactivity with the purified polyclonal IgG antibody elicited against the corresponding immunogen as measured by SPR. Sequence variations in 089CON and PB7 MPER compared with HxB2 MPER are color-coded in orange (089CON) and blue (PB7). Data are representative of three independent measurements varying peptide or antibody amounts.