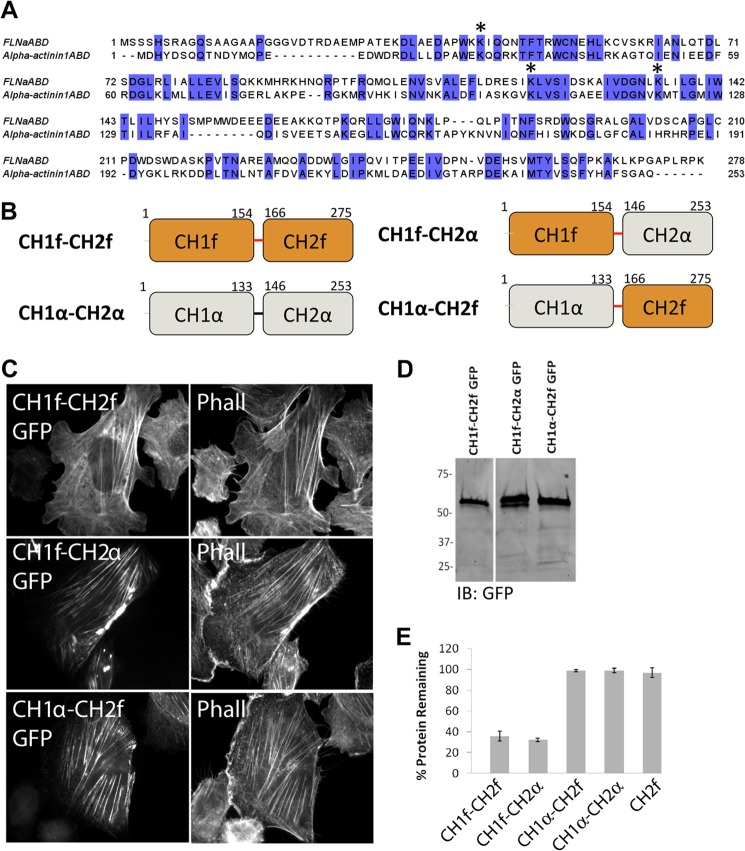
FIGURE 7.
ASB2α targets ABD chimera CH1f-CH2α, but not CH1α-CH2, for degradation. A, sequence alignment of human FLNaABD and α-actinin1ABD. The sequences are aligned with ClustalW2 and colored by sequence identity. Conserved lysine residues within CH1 are denoted by stars. B, schematic representation of FLNaABD (CH1f-CH2f), α-actinin1ABD (CH1α-CH2α), CH1α-CH2f (aa 1–133 of α-actinin1 plus aa 166–275 of FLNa), and CH1f-CH2α (aa 1–154 of FLNa plus aa 146–253 of α-actinin1) chimeras both comprising the linker region of FLNa (aa 155–165). C, CHO cells transfected with CH1f-CH2f GFP, CH1f-CH2α GFP, and CH1α-CH2f GFP were fixed and stained for phalloidin (Phall). D, cells from C were lysed and immunoblotted (IB) using anti-GFP antibody. E, CHO cells transiently expressing CH1f-CH2f (FLNaABD) GFP, CH1f-CH2α GFP and CH1α-CH2f GFP, CH1α-CH2α (α-actinin1ABD) GFP, and CH2 GFP were transfected with either dsRed-ASB2α or dsRed-ASB2αΔS, and protein degradation was assessed as in Fig. 1. Bar chart depicts mean percentage of GFP-tagged protein remaining ± S.E. in dsRed-ASB2α-expressing cells normalized to levels in dsRed-ASB2αΔS expressing cells (see “Experimental Procedures” for details). Data are from at least three independent experiments.