Abstract
Background. We estimated the 72-month efficacy of medical male circumcision (MMC) against herpes simplex virus 2 (HSV-2) incidence among men in the Kisumu MMC randomized trial.
Methods. From 2002 to 2005, 2784 men aged 18–24 were enrolled and randomized 1:1 to immediate circumcision or control. Cox proportional hazards regression incorporating stabilized inverse probability of treatment and censoring weights generated through marginal structural modeling was used to estimate the efficacy of MMC on HSV-2 risk. Conventional conditional Cox regression identified multivariable risks for HSV-2 seroconversion.
Results. Among 2044 HSV-2 seronegative men at baseline, the cumulative 72-month HSV-2 incidence was 33.5% (32.7% among circumcised men, 34.6% among uncircumcised men). In weight-adjusted Cox regression, the hazard ratio was 0.88 (95% confidence interval, .77–1.10). In multivariable analyses, risks for HSV-2 included human immunodeficiency virus (HIV) infection, genital ulcer disease (GUD), penile epithelial trauma, multiple recent sex partners, and being married /cohabiting.
Conclusions. MMC had no effect on acquisition of HSV-2 during 72 months of follow-up. The temporal sequence and limited correlation between HSV-2, GUD, and penile epithelial trauma suggests that these are distinct phenomena rather than misclassification of HSV-2 symptoms. Determining the etiology of non–sexually transmitted infection GUD and penile epithelial trauma is needed, as both are commonly occurring risks for HSV-2 and HIV acquisition.
Clinical Trials Registration. NCT0005937.
Keywords: male circumcision, HSV-2, HIV, genital ulcer disease, penile epithelial injury, Kenya
Three randomized controlled trials have shown that medical male circumcision (MMC) reduces the risk of human immunodeficiency virus (HIV) acquisition in heterosexual men by approximately 60% [1–3], and this protective effect is maintained several years after circumcision [4, 5]. The World Health Organization recommends MMC as an important element of HIV prevention programs [6]. One of the mechanisms by which MMC reduces risk of HIV seroconversion is through reduction of cofactors, such as herpes simplex virus 2 (HSV-2). In the MMC trial in Rakai, Uganda, circumcision resulted in a 28% (95% confidence interval [CI], 8%–44%) reduction in HSV-2 acquisition [7], similar to the 30% (95% CI, 1%–51%) reduction in HSV-2 incidence observed among Orange Farm, South Africa, MMC trial participants [8]. The extent to which MMC protects against HIV through reduction of HSV-2 is important for understanding how to maximize HIV prevention among men seeking circumcision and those choosing to remain uncircumcised. Among Orange Farm trial participants, authors estimated that 28% (95% CI, 18%–37%) of HIV infections may have been due to HSV-2 infection [8], and 11% (95% CI, 5%–38%) in the Rakai trial [9]. In contrast, in our trial in Kisumu, Kenya, at 24 months of follow-up, MMC was not protective against HSV-2, with an adjusted hazard ratio (HR) of 0.94 (95% CI, .70–1.25) [10]. To further understand the discrepancy in findings from our trial and the Ugandan and South African MMC trials, we assessed the efficacy of MMC against HSV-2 incidence under posttrial surveillance conditions, and examined independent risks for HSV-2 acquisition.
METHODS
This study was approved by the institutional review boards of the University of Illinois at Chicago, the Kenyatta National Hospital (Kenya), RTI International, and the University of Manitoba, and was overseen by a data and safety monitoring board. During 2002–2005, the MMC trial in Kisumu enrolled 2784 men aged 18–24 years. For inclusion, men had to (1) be uncircumcised, HIV negative, and sexually active in the previous 12 months; (2) have a hemoglobin level >9.0 g/dL; and (3) reside in Kisumu district. Exclusion criteria included foreskin covering less than half of the glans, a bleeding disorder, keloid formation, other condition(s) that might increase the risks of elective surgery, or a medical indication for circumcision. Participants with sexually transmitted infections (STIs) or other treatable medical conditions were deferred until treated. Trial recruitment, enrollment, reasons for refusing enrollment, and follow-up have been previously described [1]. Following written informed consent, participants were randomized 1:1 to either immediate circumcision or control (delayed circumcision after a 2-year follow-up period).
Both groups underwent STI and HIV risk reduction counseling and were provided unlimited supplies of free condoms. Detailed evaluations were conducted at baseline, and every 6 months from randomization for all men. At each visit, participants underwent a standardized medical history and physical examination. For this analysis, genital ulcer disease (GUD) was defined as clinically detected ulcers or self-reported genital ulcers occurring in the past 6 months. We did not use a restricted definition of clinically detected GUD, and clinicians were asked to record any epithelial defect. For planned visits occurring 6 months from randomization or later, participants underwent personal interviews to obtain sociodemographic information, measures of sexual behavior and genital hygiene practices, and attitudes toward circumcision. At each visit, men were asked 3 questions about penile injuries occurring in the past 6 months: penis feels sore during sex; penis gets scratches, cuts, or abrasions during sex; skin of the penis bleeds after sex.
HIV Testing
Testing for HIV infection was conducted using a parallel double rapid test protocol, using Determine HIV 1/2 (Abbott Diagnostic Division) and the Uni-Gold Recombigen HIV Test (Trinity Biotech). Men with concordant negative test results were eligible for the study. Concordant positive results were confirmed by double enzyme-linked immunosorbent assay (ELISA), and men were informed of their HIV status and followed up at the study clinic or referred for care to the New Nyanza Provincial Hospital or other facilities of their choice. Men with discordant results were followed up with additional tests to determine their HIV status, but were not enrolled. For confirmation of HIV seroconversion, positive rapid test and ELISA test results were done by Health Canada's National HIV Reference Laboratory (Ottawa, Canada) by line immunoassay (INNO-LIA HIV 1/2, Immunogenetics NV). Specimens indeterminate by line immunoassay were tested by polymerase chain reaction (PCR) at Health Canada or the Fred Hutchinson Cancer Research Center (Seattle, Washington), with the PCR result deemed to be definitive.
Sexually Transmitted Infection Testing
STI testing methods have been reported in detail previously [11]. In brief, men were tested for urogenital infection with Neisseria gonorrhoeae, Chlamydia trachomatis, and Trichomonas vaginalis at baseline and at each planned 6-month study visit, and at interim visits where symptoms or signs of infection were elicited. Specimens were collected for HSV-2 testing at baseline prior to randomization and at every 6-month planned study visit thereafter. Serum specimens were tested for HSV-2 antibody (Kalon HSV-2 IgG ELISA, Kalon Biological Ltd), using the manufacturer's recommended cutoff. After baseline specimen testing, the last available sample was tested in men who were initially HSV-2 seronegative. If the last available sample was HSV-2 seropositive, then previous samples were tested to determine the visit at which HSV-2 seroconversion occurred. Active syphilis infection was defined as positive rapid plasma reagin (Macro-Vue, Becton Dickinson) in conjunction with positive Treponema pallidum hemagglutination assay (Randox Laboratories Ltd). Syphilis incidence was rare (0.8%) and did not differ by circumcision status (HR = 1.09); it was not included in inferential analyses but is presented descriptively.
Analytic Sample
The trial was stopped by the data and safety monitoring board at the third interim analysis on 12 December 2006. Of the 1740 men still enrolled and eligible to participate in extended follow-up, 1545 (89%) consented to do so, with 50.3% initially randomized to delayed circumcision. The baseline prevalence of HSV-2 in this extended follow-up sample was 27.2%, similar to the 28% baseline prevalence of HSV-2 among the initial 2784 men included in the trial [1]. The 421 men who were HSV-2 seropositive at baseline were excluded from this analysis. Of the remaining 1124 men, 570 (50.7%) were initially randomized to the control group. This analysis additionally excluded 3 HIV-uninfected men who were found to be outside the inclusion age range. The follow-up visit schedule, personal interview, physical examination, and STI and HIV testing procedures were identical to those of the trial, with scheduled visits every 6 months. Extended follow-up was completed on 30 September 2010, and observation ceased at that time.
Marginal Structural Model Analysis
To address the concern of time-varying confounding, we evaluated the effectiveness of MMC on HSV-2 risk over extended follow-up using marginal structural models. For each individual, at each follow-up, we estimated the probability of becoming circumcised conditional on fixed and time-varying covariates up to that time. These weights effectively balance the distribution of potential confounders, reducing the bias introduced by self-selection to become circumcised after trial end, and enabling an approximation of the causal association between circumcision status and HSV-2 risk [12]. For our marginal structural approach, we generated inverse probability of treatment weights (IPTWs) to achieve greater efficiency and coverage in 95% CIs for the association between circumcision status and HSV-2 incidence [13]. We extended these weights to censoring, and similarly calculated stabilized inverse probability of censoring weights (IPCWs) to account for time-dependent loss to follow-up. Weights were obtained through fitting a weighted pooled logistic regression model for circumcision status (or censoring) using each study visit as an observation. Baseline and time-varying predictors of becoming circumcised or being lost to follow-up (censored) were selected a priori based on a conceptual framework to maintain statistical efficiency [13]. Following methods by Hernán et al, the overall weights for subjects were calculated as the product of stabilized treatment and censoring weights [13]. These IPTW*IPTC weights were applied to the observed data, and an unadjusted model for HSV-2 incidence as a function of circumcision status was fitted to the weighted sample. This unadjusted, weighted logistic model was fitted using generalized estimating equations assuming an exchangeable correlation structure, with robust variance estimation.
To identify risks for HSV-2 acquisition, we used a conventional Cox regression model to show HSV-2 incidence as a function of covariates of interest. Circumcision status was analyzed as a time-varying covariate by status (as treated). The assumption of proportionality for Cox proportional hazards was confirmed by testing for a nonzero slope in a generalized linear regression of the scaled Schoenfeld residuals on functions of time. Standard errors were estimated using a robust variance estimate. Data were analyzed using Stata/SE 11.2 for Windows (StataCorp).
RESULTS
As previously reported, the control and treatment arms were well balanced at baseline regarding sociodemographic and behavioral characteristics, STI prevalences, and rates of follow-up [1]. Comparisons are similar when restricted to men who were HSV-2 seronegative at baseline (Table 1).
Table 1.
Selected Baseline Sociodemographic Characteristics and Behaviors by Treatment Assignment, Among Men Who Were Herpes Simplex Virus 2–Seronegative at Baseline
| Characteristica | Circumcision Group (n = 1001), No. (%) | Control Group (n = 1043), No. (%) |
|---|---|---|
| Reported age, y | ||
| 18–20 | 529 (52.9) | 525 (52.9) |
| 21–24 | 472 (47.1) | 491 (47.1) |
| Marital status | ||
| Not married or living with a female sex partner | 956 (95.8) | 981 (94.6) |
| Married or living with a female sex partner | 42 (4.2) | 56 (5.4) |
| No. of sex partners in the past 30 db | ||
| 0 | 430 (43.0) | 499 (48.0) |
| 1 | 395 (39.5) | 392 (37.7) |
| ≥2 | 175 (17.5) | 148 (14.2) |
| No. of sex partners in the past 6 mo | ||
| 0 | 141 (14.1) | 157 (15.1) |
| 1 | 445 (44.5) | 475 (45.7) |
| ≥2 | 413 (41.3) | 407 (39.2) |
| Used a condom the last time you had sexual intercourse | ||
| No | 486 (48.6) | 526 (50.6) |
| Yes | 514 (51.4) | 513 (49.4) |
| How many hours until you washed your penis after the last time you had sex? | ||
| <1 h | 233 (23.5) | 224 (21.7) |
| ≥1 h | 760 (76.5) | 809 (78.3) |
| Endorsement of circumcision score, median (95% CI)c | 3 (3–3) | 3 (3–3) |
| Self-reported scratches, cuts, abrasions, or bleeding of skin of penis after sex in the past 6 mo | ||
| No | 526 (52.7) | 566 (54.6) |
| Yes | 473 (47.3) | 471 (45.4) |
| Painless or painful genital ulcer in past 6 mo or currently (by report), or ulcer on examinationb | ||
| No | 969 (96.8) | 1025 (98.3) |
| Yes | 32 (3.2) | 18 (1.7) |
| Nonulcerative sexually transmitted infection | ||
| Neisseria gonorrhoeae | 17 (1.7) | 15 (1.4) |
| Chlamydia trachomatis | 47 (4.7) | 32 (3.1) |
| Trichomonas vaginalis | 16 (1.6) | 21 (2.0) |
| Infection with any of the above | 74 (7.4) | 60 (5.8) |
a Sample sizes vary slightly by characteristic due to a few missing responses.
b χ2 P < .05.
c 95% CI is binomially obtained.
Over the extended follow-up period, approximately 50% of men randomized to delayed circumcision became circumcised (n = 258), with 32% (n = 82) becoming circumcised within 12 months of the end of the trial. Men who at baseline were married or cohabiting or who had urethral discharge were more likely to become circumcised (Table 2). Condom use at baseline was associated with an increased likelihood of becoming circumcised, whereas condom use over follow-up was associated with a decreased likelihood of becoming circumcised. Unsurprisingly, increasing endorsement of circumcision over follow-up was associated with a higher likelihood of becoming circumcised. GUD at baseline or over follow-up, and penile epithelial injury over follow-up, had a strong inverse association with becoming circumcised.
Table 2.
Results of Logistic Regression Models to Generate Weights for Circumcision and Censoringa
| Variables | Circumcision, OR (95% CI) | Censoring, OR (95% CI) |
|---|---|---|
| Baseline covariates | ||
| Age 21–24 y (vs 18–20 y) | 1.08 (.90–1.28) | 1.04 (.91–1.19) |
| Highest completed educational attainment | ||
| None, primary 1–8 | Ref | Ref |
| Some secondary | 1.08 (.84–1.38) | 0.96 (.91–1.19) |
| Secondary or higher | 1.12 (.91–1.39) | 1.08 (.92–1.26) |
| Married or cohabiting (vs not married and not cohabiting) | 2.30 (1.53–3.46) | 1.40 (1.01–1.94) |
| Resides in Kisumu district (vs other district) | 1.01 (.73–1.39) | 0.79 (.61–1.04) |
| Income source | ||
| None | Ref | Ref |
| Self-employed | 0.90 (.65–1.24) | 1.01 (.81–1.27) |
| Salaried | 0.98 (.76–1.28) | 0.94 (.76–1.18) |
| Condom used at last intercourse (yes vs no) | 1.99 (1.66–2.37) | 0.96 (.84–1.09) |
| No. of sex partners in past 6 mo | ||
| 0 | Ref | Ref |
| 1 | 0.78 (.60–1.02) | 0.90 (.74–1.08) |
| ≥2 | 0.71 (.60–1.02) | 0.83 (.68–1.01) |
| Endorsement of circumcision | 1.03 (.97–1.09) | 1.05 (1.01–1.10) |
| Self-reported or clinically detected GUD | 0.11 (.01–.83) | 1.34 (.84–2.14) |
| Urethral discharge detected on exam or by self-report | 2.00 (1.30–3.06) | 0.77 (.53–1.12) |
| Self-reported scratches, cuts, abrasions, bleeding of skin of penis after sexual intercourse, occurring in the past 6 mo | .96 (.81–1.14) | 1.04 (.92–1.19) |
| Time-varying covariates | ||
| Circumcised | 0.94 (.82–1.08) | |
| Married or cohabiting (vs not married and not cohabiting) | 0.91 (.73–1.14) | 0.76 (.64–.90) |
| Resides in Kisumu district (vs other district) | 1.01 (.73–1.40) | 0.88 (.68–1.15) |
| Income source | ||
| None | Ref | Ref |
| Self-employed | 0.83 (.68–1.03) | 0.70 (.60–.82) |
| Salaried | 0.99 (.76–1.28) | 0.81 (.68–.97) |
| Condom used at last intercourse (yes vs no) | 0.77 (.63–.94) | 0.93 (.81–1.08) |
| No. of sex partners in past 6 mo | ||
| 0 | Ref | Ref |
| 1 | 1.02 (.78–1.34) | 0.93 (.76–1.12) |
| ≥2 | 1.20 (.90–3.08) | 0.90 (.73–1.11) |
| Endorsement of circumcision | 1.43 (1.35–1.51) | 0.91 (.88–.95) |
| Self-reported or clinically detected GUD | 0.30 (.10–.90) | 0.81 (.41–1.60) |
| Urethral discharge detected on exam or by self-report | 1.62 (.86–3.08) | 1.03 (.62–1.70) |
| Self-reported scratches, cuts, abrasions, bleeding of skin of penis after sexual intercourse, occurring in the past 6 mo | 0.59 (.47–.75) | 1.10 (.93–1.30) |
Abbreviations: CI, confidence interval; GUD, genital ulcer disease; HSV-2, herpes simplex virus 2; OR, odds ratio.
a Models presented are the denominators for the stabilized weights: pooled logistic regression models for circumcision and for censoring.
Posttrial retention among men consenting to extended follow-up who were HSV-2 seronegative at baseline was 87% at 36 months, 78% at 48 months, 67% at 60 months, and 54% at 72 months. Censoring by loss to follow-up was more likely among men who, at baseline, were married/cohabiting and had greater endorsement of circumcision. There was a marginally statistically significant inverse association between having ≥2 recent sex partners or residing in Kisumu district at baseline and being lost to follow-up. Men who over follow-up were married/cohabiting, employed, or had greater endorsement of circumcision were less likely to be lost to follow-up.
MMC and HSV-2 Incidence
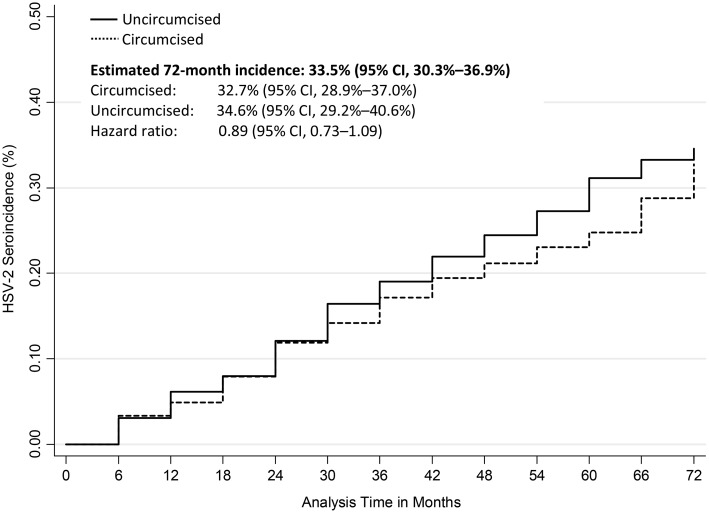
The cumulative 72-month HSV-2 incidence was 33.5% (95% CI, 30.3%–36.9%): 32.7% among circumcised men, 34.6% among uncircumcised men (Figure 1). The crude HR was 0.89 (95% CI, .73–1.09; Table 3). The results of Cox regression incorporating weights from the marginal structural model (Table 3) indicated a similar lack of protective efficacy of MMC against HSV-2 acquisition over 6 years of observation (HR = 0.88; 95% CI, .77–1.10).
Figure 1.
Cumulative herpes simplex virus 2 (HSV-2) incidence across follow-up visits by circumcision status. Abbreviation: CI, confidence interval.
Table 3.
Male Circumcision Status and Risk of Herpes Simplex Virus 2 Incidence
| Variables | Conventional Cox Model Unadjusted, HR (95% CI) | Cox Regression with Weights From Marginal Structural Modelinga, | Conventional Cox Model Adjusted for Time-Varying Factors, |
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | ||
| Circumcised | 0.89 (.73–1.09) | 0.88 (.77–1.10) | 1.00 (.82–1.22) |
| Self-reportedb or clinically detected GUD | 4.75 (3.02–7.48) | ||
| HIV positive | 3.75 (2.55–5.52) | ||
| Skin of penis ever gets scratches during or after sexb | 1.47 (1.18–1.83) | ||
| Number of sex partners in the past 30 d | |||
| 0 | Ref | ||
| 1 | 1.27 (.99–1.62) | ||
| ≥2 | 1.54 (1.14–2.08) | ||
| Married or cohabiting | 1.66 (1.34–2.07) | ||
| Lives in Kisumu district | 1.24 (1.02–1.50) | ||
All covariates are time-varying.
Abbreviations: CI, confidence interval; GUD, genital ulcer disease; HIV, human immunodeficiency virus; HR, hazard ratio; HSV-2, herpes simplex virus 2.
a Weighted marginal structural model is adjusted for month of visit (5-knot cubic spline with knots at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles).
b Recall period is past 6 months.
A conventional multivariable Cox regression model Table 3 adjusted for all other variables significant at the P < .05 level echoed the null association between MMC and HSV-2 acquisition (adjusted HR [AHR] = 1.00; 95% CI, .82–1.22). HIV seroconversion was a strong risk for HSV-2 acquisition (AHR = 3.75; 95% CI, 2.55–5.52). Other factors associated with HSV-2 seroincidence included genital ulcer disease (AHR = 4.75; 95% CI, 3.02–7.48); self-reported scratches, cuts, or abrasions of the penile skin during or after sex (AHR = 1.47; 95% CI, 1.18–1.83); and having ≥2 sex partners in the past 30 days (AHR = 1.54; 95% CI, 1.14–2.08). Living in Kisumu and being married/cohabiting also carried increased risk of HSV-2 acquisition (AHR = 1.24 and 1.66, respectively). There were no statistically significant or meaningful 2-way interaction terms between any of the statistically significant variables or circumcision status.
Sequence of Events: HSV-2 Incidence, GUD, Penile Epithelial Disruption
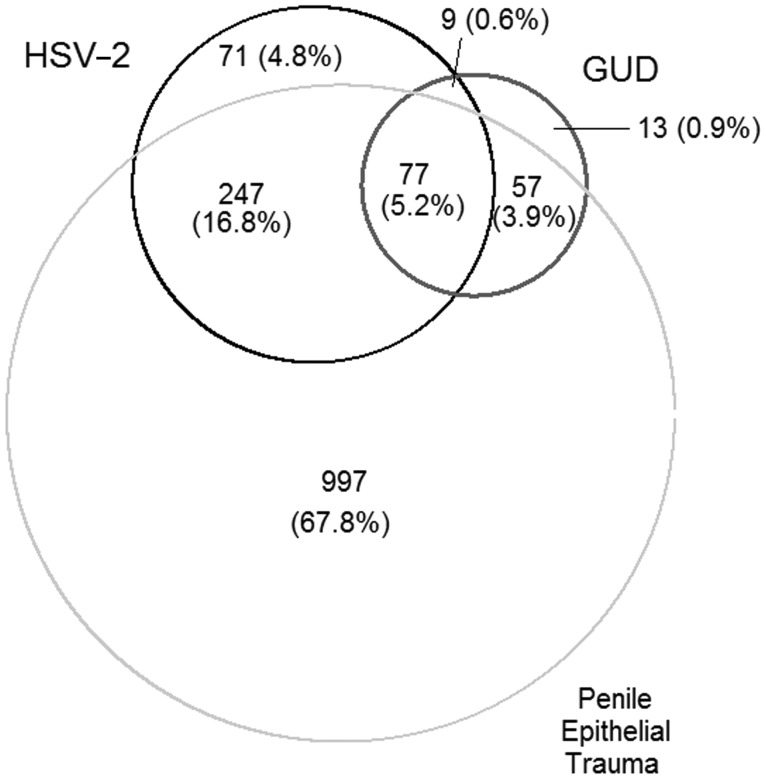
Of the 404 men with incident HSV-2, 18 (4.5%) experienced a first occurrence of GUD at the same visit that HSV-2 was first detected. HSV-2 seroincidence was preceded by GUD at any time interval in 51 (12.6%) men, and the median time from GUD to HSV-2 seroincidence was 12 months (95% CI, 6.2–18 months). For 17 (4.2%) individuals, a first occurrence of GUD followed HSV-2 seroincidence by at least 6 months, with a median time from HSV-2 to first GUD occurrence of 18 months (95% CI, 12–24 months). Therefore, 318 (78.7%) men with seroincident HSV-2 did not experience GUD over follow-up (Figure 2). Overall, 156 men had GUD; 70 (44.9%) remained HSV-2 seronegative over follow-up, and 86 (55.1%) experienced GUD prior to, at the same time as, or after HSV-2 incidence (as described above). Of the 70 men with GUD who remained HSV-2 seronegative over follow-up, 5 (7.1%) had active syphilis.
Figure 2.
Proportional Venn diagram depicting the relationship between herpes simplex 2 (HSV-2), genital ulcer disease (GUD), and penile epithelial trauma (defined as self-reported scratches, cuts, abrasions, or bleeding of the penile skin occurring during or after sex in the past 6 months). Area-proportional Venn diagram showing the relative frequency and overlap of HSV-2 (n = 404), GUD (n = 156), and penile epithelial trauma (n = 1378). Numbers represent unique subjects: overlap represents events occurring within a single subject observed at the same time (simultaneous) or at different time points. Percentages reflect the proportion of the total number of men (N = 1471) with HSV-2, GUD, or penile epithelial trauma.
Among 1378 (67.4%) men who reported scratches, cuts, abrasions, or bleeding of the penile skin during or after intercourse, 324 (23.5%) acquired HSV-2 (Figure 2). Of the 404 men with incident HSV-2, 25 (6.2%) experienced a first occurrence of penile epithelial disruption at the same time that HSV-2 was first detected. For 20 (5.0%) individuals, HSV-2 seroincidence preceded penile epithelial disruption, with a median time from HSV-2 to trauma of 18 months (95% CI, 6–29 months). For 279 (69.1%) men, penile epithelial disruption preceded HSV-2 seroincidence, with a median time from trauma to HSV-2 of 24 months (95% CI, 18–24 months). In large part this is because 904 of the 1378 (65.6%) men reporting penile scratches, cuts, or abrasions did so at baseline. Therefore, 80 (19.8%) men with seroincident HSV-2 did not experience penile scratches, cuts, abrasions, or bleeding (Figure 2). Among men with penile skin trauma, 15 were also diagnosed with active syphilis. In 3 cases, syphilis preceded reported penile skin trauma; in 5 cases syphilis and penile skin trauma occurred at the same visit; and in 7 cases penile skin trauma preceded syphilis.
DISCUSSION
Accounting for differences in who becomes circumcised and who is lost to follow-up, MMC had no effect on HSV-2 acquisition over 6 years of observation, virtually the same as the findings observed under trial conditions [10].
These findings are in contrast to those of the Ugandan and South African MMC trials, which found a 28%–30% protective effect of MMC against HSV-2 acquisition [8, 9]. Tobian et al posited that our null finding observed at 24 months under trial conditions was likely due to lack of specificity of the HSV-2 assay used [14]. However, our sensitivity analyses varying the optical density index cutpoint for determination of positives demonstrated that this does not explain the lack of an association [10, 15]. Therefore, it is unlikely that the contrasting findings are due to differences in detection of HSV-2. Alternatively, there are substantial cohort-level and individual-level differences between the trials that may have affected the results. The men enrolled in the Kisumu and Orange Farm trials were aged 18–24 years, whereas men enrolled in the Rakai trial were aged 15–49 years. The prevalence of HSV-2 at baseline was low in the Orange Farm trial (5.9%) [16], compared to 17% among Rakai participants aged 15–24 years [17] and 27% among Kisumu participants. In the Rakai trial, risk for HSV-2 acquisition was 35% lower among men aged 30–49 years compared to those aged 15–19 years [17]. At baseline, Kisumu participants had more sex partners over their lifetime and in the past 12 months than Rakai participants aged 18–24 or Orange Farm participants [18]. Our marginal structural model–weighted HR of 0.88 (95% CI, .77–1.10) is in keeping with the results of the meta-analysis by Weiss et al [19], who found a summary relative risk for the association between MMC and HSV-2 of 0.88 (95% CI, .77–1.01). It is possible that the higher prevalence of infection and greater risk of exposure for younger men in Kisumu overwhelmed any potential moderate or small protective effect of MMC against HSV-2.
In our conventional multivariable Cox regression model, GUD and penile epithelial trauma were risks for HSV-2 acquisition. Had this been misclassification of herpetic lesions, we would have expected greater proportional and temporal correlation between variables. Although 21% of men with HSV-2 also experienced GUD, and 80% experienced penile epithelial trauma, 45% of men with GUD and 80% of men reporting penile epithelial trauma did not acquire HSV-2. Moreover, the temporal sequence does not support misclassification, with GUD preceding HSV-2 in 59% of men with both conditions, and penile epithelial trauma preceding HSV-2 in 92% of men with both conditions. In addition to epidemiologic evidence, biologic evidence supports nonherpetic GUD and penile epithelial trauma as phenomena distinct from each other and from HSV-2. Approximately 60% of clinically detected GUD in our trial was not associated with HSV-2 or syphilis by PCR and serology [20]. Rather, pyrosequencing of clinically detected genital ulcers demonstrated that such non-STI GUD was associated with specific anaerobic bacteria that have cytotoxic and tissue-destructive properties. Non-STI GUD and penile epithelial disruption may increase the risk of HSV-2 acquisition through disruption of the mucosal barrier facilitating HSV-2 penetration or by altered local immunity. As we previously reported, controlling for circumcision status, penile epithelial trauma is a risk factor for HIV acquisition [13], and MMC reduces the risk of scratches, cuts, and abrasions to the penile skin by approximately 50% [21]. Further study is needed to characterize the etiology of non-STI GUD and penile epithelial trauma because of their association with HIV [13], and because of the high frequency observed among men in our cohort and in other populations in sub-Saharan Africa [22, 23].
HIV acquisition led to a >3-fold increase in the risk of HSV-2 acquisition. Our findings are in keeping with those from the Orange Farm MMC trial, where Sobngwi-Tambeku et al report that men who were HIV seropositive at baseline had a 2.5-fold increase in risk of HSV-2 acquisition [8]. HIV may increase risk of HSV-2 acquisition through shared behavioral risks [24] or impaired genital immune control [25]. Our results reinforce the importance of continued focus on preventing GUD and HSV-2 acquisition among HIV-infected persons as both increase HIV transmissibility [26, 27] and as HSV-2 accelerates HIV progression [28, 29].
The increased risk of HSV-2 associated with residence in Kisumu district may represent a higher population prevalence in this more urban district compared to surrounding districts, which are less urbanized and less densely populated. Although not a modifiable risk, it is important for planning screening and outreach programs. Men who were married or cohabiting had increased risk of HSV-2 acquisition, as did men with greater numbers of recent sex partners; these risks are in keeping with our previous analyses of risks for prevalent HSV-2 infection and for prevalent and incident gonorrhea and chlamydia [11]. Population-based surveillance estimates that 5.8% of married Kenyan couples are HIV discordant, representing >300 000 individuals [30]. In the context of high HSV-2 prevalence and incidence, and high risk of HIV acquisition, married couples should be a priority population for HSV-2 prevention and control.
To the extent possible, our models were developed to minimize unmeasured confounding, nonpositivity, and misspecification. Our IPTW (mean, 1.00; range, 0.41–3.10), IPCW (mean, 1.00; range, 0.35–3.78), and IPTW*IPCW (mean, 0.99; range, 0.38–3.37) were centered at “1” with narrow ranges, suggesting that nonpositivity and misspecification were not significant concerns [12]. The men in our analysis were members of a long-term cohort, with regular counseling and testing for STI and HIV risk reduction, and promotion of medical male circumcision, which may affect generalizability of results.
In summary, MMC did not reduce HSV-2 acquisition after 72 months of follow-up, similar to findings of our trial under conditions of randomization at 24 months of follow-up. Reasons for the divergence of our trial findings and those of the other trials of MMC should be investigated to determine effective approaches for HSV-2 prevention. Effective prevention of nonherpetic GUD and genital epithelial disruption may lead to reduced risk of HSV-2 and associated HIV risk.
Notes
Author contributions. Study concept and design: R. C. B., S. M. Acquisition of data: K. A., R. C. B., I. M., S. M., E. O.-J. Drafting of the manuscript: S. D. M. Critical revision of the manuscript for important intellectual content: K. A., R. C. B., H. L., I. M., S. D. M., S. M., E. O.-J. Statistical analysis: H. L., S. D. M. Analysis and interpretation of data: H. L., S. D. M. Obtained funding: R. C. B., S. M. Administrative, technical, or material support: K. A., R. C. B., I. M., S. D. M., S. M., E. O.-J. The corresponding author (S. D. M.) had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, Division of AIDS, National Institutes of Health (grant number AI50440) and by the Canadian Institutes of Health Research (grant number HCT 44180). R. C. B. was partially supported by the Chicago Developmental Center for AIDS Research, a National Institutes of Health–funded program (P30 AI 082151).
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369:643–56. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 2.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–66. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 3.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. Epub 25 October 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta SD, Moses S, Agot K, et al. The long term efficacy of medical male circumcision against HIV acquisition. AIDS 2013 [Epub ahead of print] doi: 10.1097/01.aids.0000432444.30308.2d. [DOI] [PubMed] [Google Scholar]
- 5.Gray R, Kigozi G, Kong X, et al. The effectiveness of male circumcision for HIV prevention and effects on risk behaviors in a posttrial follow-up study. AIDS. 2012;26:609–15. doi: 10.1097/QAD.0b013e3283504a3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. New data on male circumcision and HIV prevention: policy and programme implications. WHO/UNAIDS technical consultation. Male circumcision and HIV prevention: research implications for policy and programming: conclusions and recommendations; 6–8 March 2007; Montreux: http://www.who.int/hiv/mediacentre/MCrecommendations_en.pdf. Accessed 28 September 2012. [Google Scholar]
- 7.Tobian AAR, Serwadda D, Quinn TC, et al. Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Engl J Med. 2009;360:1298–309. doi: 10.1056/NEJMoa0802556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sobngwi-Tambekou J, Taljaard D, Lissouba P, et al. Effect of HSV-2 serostatus on acquisition of HIV by young men: results of a longitudinal study in Orange Farm, South Africa. J Infect Dis. 2009;199:958–64. doi: 10.1086/597208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray RH, Serwadda D, Tobian AAR, et al. Effects of genital ulcer disease and herpes simplex virus type 2 on the efficacy of male circumcision for HIV prevention: analyses from the Rakai trials. PLoS Med. 2009;6:e1000187. doi: 10.1371/journal.pmed.1000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta SD, Moses S, Parker CB, Agot K, Maclean I, Bailey RC. Circumcision status and incident HSV-2 infection, genital ulcer disease, and HIV infection. AIDS. 2012;26:1141–9. doi: 10.1097/QAD.0b013e328352d116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta SD, Moses S, Agot K, et al. Adult male circumcision does not reduce risk of incident Neisseria gonorrhoeae, Chlamydia trachomatis, and Trichomonas vaginalis: results from a randomized controlled trial in Kenya. J Infect Dis. 2009;200:370–8. doi: 10.1086/600074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–64. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–70. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Tobian AA, Kigozi G, Wawer MJ, Serwadda D, Quinn TC, Gray RH. Herpes simplex virus type-2 assay specificity and male circumcision to reduce herpes simplex virus type-2 acquisition. AIDS. 2013;27:147–9. doi: 10.1097/QAD.0b013e32835aa181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta SD, Moses S, Parker CB, Agot K, Maclean I, Bailey RC. Response to ‘herpes simplex virus type-2 (HSV-2) assay specificity and male circumcision to reduce HSV-2 acquisition. AIDS. 2013;27:149–50. doi: 10.1097/QAD.0b013e328358cc92. [DOI] [PubMed] [Google Scholar]
- 16.Mahiane SG, Legeai C, Taljaard D, et al. Transmission probabilities of HIV and herpes simplex virus type 2, effect of male circumcision and interaction: a longitudinal study in a township of South Africa. AIDS. 2009;23:377–83. doi: 10.1097/qad.0b013e32831c5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tobian AAR, Charvat B, Ssempijja V, et al. Factors associated with the prevalence and incidence of herpes simplex virus type 2 infection among men in Rakai, Uganda. J Infect Dis. 2009;1997:945–9. doi: 10.1086/597074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta SD, Gray RH, Auvert B, et al. Does sex in the early period after circumcision increase HIV-seroconversion risk? Pooled analysis of adult male circumcision clinical trials. AIDS. 2009;23:1557–64. doi: 10.1097/QAD.0b013e32832afe95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiss HA, Thomas SL, Munabi SK, Hayes RJ. Male circumcision and risk of syphilis, chancroid, and genital herpes: a systematic review and meta-analysis. Sex Transm Infect. 2006;82:101–9. doi: 10.1136/sti.2005.017442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta SD, Green S, Maclean I, et al. Microbial diversity of genital ulcer disease in men enrolled in a randomized trial of male circumcision in Kisumu, Kenya. PLoS One. 2012;7:e38991. doi: 10.1371/journal.pone.0038991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta SD, Krieger JN, Agot K, et al. Circumcision and reduced risk of self-reported coital injuries: results from a randomized controlled trial in Kisumu, Kenya. J Urol. 2010;184:203–9. doi: 10.1016/j.juro.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailey RC, Neema S, Othieno R. Sexual behaviors and other HIV risk factors in circumcised and uncircumcised men in Uganda. J Acquir Immune Defic Syndr. 1999;22:294–301. doi: 10.1097/00126334-199911010-00012. [DOI] [PubMed] [Google Scholar]
- 23.Kalichman SC, Simbayi LC, Cain D, Cherry C, Jooste S. Coital bleeding and HIV risks among men and women in Cape Town, South Africa. Sex Transm Dis. 2006;33:551–7. doi: 10.1097/01.olq.0000218868.76820.7f. [DOI] [PubMed] [Google Scholar]
- 24.Tassiopoulos KK, Seage G, III, Sam N, et al. Predictors of herpes simplex virus type 2 prevalence and incidence among bar and hotel workers in Moshi, Tanzania. J Infect Dis. 2007;195:493–501. doi: 10.1086/510537. [DOI] [PubMed] [Google Scholar]
- 25.Rebbapragada A, Wachihi C, Pettengell C, et al. Negative mucosal synergy between herpes simplex type 2 and HIV in the female genital tract. AIDS. 2007;21:589–98. doi: 10.1097/QAD.0b013e328012b896. [DOI] [PubMed] [Google Scholar]
- 26.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 27.Johnson LF, Lewis DA. The effect of genital tract infections on HIV-1 shedding in the genital tract: a systematic review and meta-analysis. Sex Transm Dis. 2008;35:946–59. doi: 10.1097/OLQ.0b013e3181812d15. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds SJ, Makumbi F, Newell K, et al. Effect of daily acyclovir on HIV disease progression in individuals in Rakai, Uganda, co-infected with HIV-1 and herpes simplex virus type 2: a randomised, double-blind placebo-controlled trial. Lancet Infect Dis. 2012;12:441–8. doi: 10.1016/S1473-3099(12)70037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mugwanya K, Baeten JM, Mugo NR, Irungu E, Nbure K, Celum C. High-dose valacyclovir HSV-2 suppression results in greater reduction in plasma HIV-1 levels compared with standard dose acyclovir among HIV-1/HSV-2 coinfected persons: a randomized, crossover trial. J Infect Dis. 2011;204:1912–7. doi: 10.1093/infdis/jir649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaiser R, Bunnell R, Hightower A, et al. Factors associated with HIV infection in married or cohabitating couples in Kenya: results from a nationally representative study. PLoS One. 2011;6:e17842. doi: 10.1371/journal.pone.0017842. [DOI] [PMC free article] [PubMed] [Google Scholar]