Abstract
Background:
The authors demonstrate the utility of indocyanine green videoangiography (ICG-VA) for intraoperative vascular flow assessment in the surgery of a variety of spinal intramedullary tumors to achieve an additional level of safety as well as precision with the surgical procedure.
Methods:
Fourteen patients with spinal intramedullary tumors (nine cervical and five thoracic) operated on between August 2011 and April 2013 were included in the present study. A fluorescence surgical microscope was used to perform ICG-VA after standard exposure of the lesion to assess the dynamic flow of the spinal microvasculature.
Results:
Twenty-seven ICG-VA injections were performed in 14 cases. Pathological diagnosis of the tumors included ependymoa, astrocytoma, cavernous malformation, or hemagioblastoma. There were no complications or side-effects related to ICG-VA. Intraoperative ICG-VA provided dynamic flow images of the spinal microvasculature in accordance with the progress of surgical procedures. Angiographic images could be divided into arterial, capillary, and venous phases. All angiographic images were well integrated into the microscopic view. The utility of ICG-VA could be summarized into three categories: (1) Localization of normal spinal arteries and veins, (2) assessment of posterior spinal venous circulation, and (3) differentiation of feeding arteries, tumor, and draining veins.
Conclusions:
Intraoperative vascular flow assessment using ICG-VA was easy, repeatable, and practical without any significant procedure-related risks. ICG-VA can be used for careful analysis of spinal microvascular flow or anatomical orientation, which is necessary to ensure safe and precise resection of spinal intramedullary tumors.
Keywords: Astrocytoma, cavernous malformation, ependymoma, hemagioblastoma, indocyanine green videoangiography, spinal intramedullary tumors
INTRODUCTION
Although surgical concept of spinal intramedullary tumors has been established and refined during the past century,[1,2,3,6,7,9,11,14,15,16,22] spinal intramedullary tumors are still the major challenge for neurosurgeons, due to their relative infrequency and unknown natural history, and difficulty in standardizing treatment. Developments in microsurgical instruments, medical equipment, and neurophysiological monitoring techniques have contributed the safety and precision of surgery. Intraoperative image guidance using near-infrared indocyanine green videoangiography (ICG-VA) has been used to provide real-time angiographic images during vascular or tumor surgery.[8,10,12,13,17,18,20] Here, we demonstrate the utility of ICG-VA for intraoperative vascular flow assessment in the surgery of spinal intramedullary tumors. Analysis of the flow dynamics of spinal microvasculature using ICG-VA would enable us to achieve an additional level of safety as well as precision with the surgical procedure.
MATERIALS AND METHODS
Patient population
Fourteen patients who underwent surgery for spinal intramedullary tumors between August 2011 and April 2013 were included in this retrospective study. There were 10 male and 4 female patients, ranging in age from 17 to 74 years (mean, 43.9 years; Table 1). The location of the tumors was cervical in nine cases and thoracic in five cases [Table 1]. All patients underwent comprehensive evaluation before and after surgery. The neurological condition before and after surgery was assessed using the modified McCormick functional and sensory pain scales [Table 2].[15,20,21] The follow-up period ranged from 4 to 23 months (mean, 9.4 months). Clinical characteristics of all patients are summarized in Table 1.
Table 1.
Characteristics of patients of spinal intramedullary tumors studied with ICG-Videoangiography
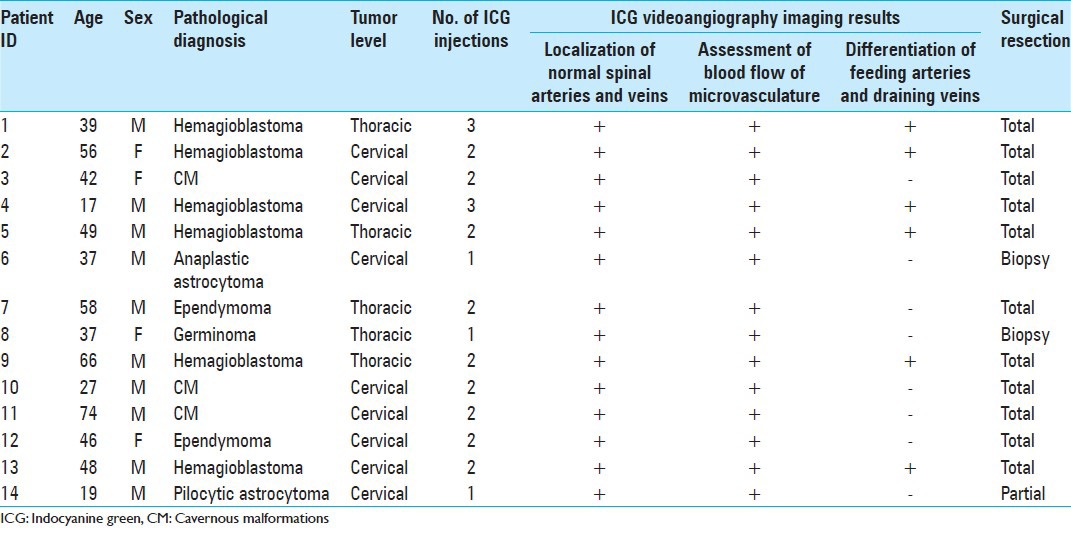
Table 2.
Modified McCormick functional schema and sensory pain scale

ICG-VA
A fluorescence surgical microscope equipped with an 800-nm observation system (Leica Microsystems GmbH, Germany) was used to perform ICG-VA after standard exposure of the lesion. ICG-VA was performed at intervals of at least 5-10 min. ICG (Daiichi Sankyo Company, Limited, Tokyo, Japan) was injected intravenously just prior to each ICG-VA. The dose of ICG was 0.1-0.3 mg/kg, with a maximum dose of 5 mg/kg/day. Written informed consent for the use of ICG was obtained from the patient prior to surgery.
Surgical technique
Our surgical technique of spinal intramedullary tumors was the same as previously described.[5,16,21] The patient was placed in the lateral oblique (45 degrees) position under general anesthesia, with the tumor located on the upper side. The thorax was elevated 15 degrees and the head was maintained in neutral flexion without any rotation. Transcranial motor evoked potentials were used for intraoperative neurophysiological monitoring.
In cases of a posterior median sulcus (PMS) or posterolateral sulcus (PLS) approach to the tumor, such as for ependymomas, astrocytomas, or cavernous malformations, posterior spinal arteries on both sides were well differentiated from posterior spinal veins or the pial venous plexus using ICG-VA [Figures 1b and 2b]. Venous circulation of posterior spinal veins or the pial venous plexus crossing just over the PMS or PLS was also assessed using ICG-VA. If stagnation or slow flow of the venous circulation of these crossing veins was suggested [Figures 1c and 2c], the veins were coagulated with a microbipolar coagulator at very low power levels under continuous saline irrigation. Otherwise, the thick posterior spinal vein was gently dissected and separated from the PMS or PLS. The PMS or PLS opening was made using a diamond knife, and the myelotomy was extended rostrally or caudally using a microdissector to meticulously splay the spinal tissue. When the tumor was encountered, gentle dissection of the tumor–cord interface was continued in the longitudinal plane over the extent of the tumor. With care to protect the adjacent dorsal and lateral columns, the tumor or capsule was removed segmentally or in one piece. In cases where the tumor–cord interface was not evident, the tumor removal was not continued. In cases of cavernous malformation, the surrounding hemosiderin stained tissue was not resected. After resection of the tumor, posterior sulcal central veins or sulcal central branches of the anterior spinal artery were analyzed using ICG-VA [Figures 1e and 2e].
Figure 1.
Intraoperative photographs from Patient 3. (a) Photographs showing a slightly swollen spinal cord. b-c. Photographs from the early phase of indocyanine green videoangiography (ICG-VA), showing the posterior spinal arteries on both sides (arrows) (b), and from the late phase of ICG-VA, showing the stagnation of venous flow (arrowheads) of posterior spinal veins and the pial venous plexus crossing veins on the posterior median sulcus (c). d-e. Photographs obtained after the complete removal of the cavernous malformations, showing the hemosiderin stained tissue surrounding the tumor (d) and well preserved posterior sulcal central veins (arrowheads) (Ee). The shape of the spinal cord was restored by suturing the pial edges together as much as possible (f)
Figure 2.
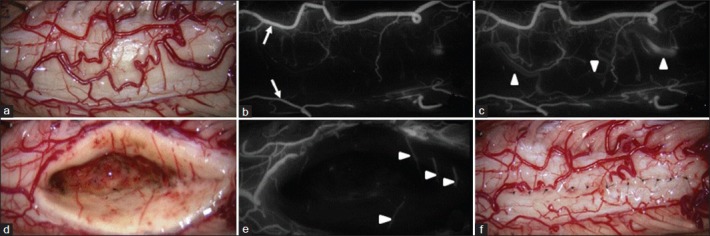
Intraoperative photographs from Patient 12. (a) Photographs showing a slightly swollen spinal cord. b-c. Photographs from the early phase of indocyanine green videoangiography (ICG-VA) showing the posterior spinal arteries on both sides (arrows) (b), and from the late phase of ICG-VA showing the stagnation of venous flow (arrowheads) of posterior spinal veins and the pial venous plexus crossing veins on the posterior median sulcus (c). d-f. Photographs obtained after the complete removal of the ependymoma, showing well preserved sulcal central branches of the anterior spinal artery (arrows) and the posterior sulcal central veins (d, e) and the surrounding gliotic tissue (f)
In cases of a transpial approach to the tumor, such as for hemangioblastomas, feeding arteries were well differentiated from draining veins using ICG-VA [Figure 3b]. Feeding arteries were interrupted just proximal to the tumor itself, and a decrease in blood flow to the tumor was confirmed using ICG-VA [Figure 3c]. Thick and cloudy pia mater encircling the tumor was cut sharply to reveal the clear gliosis layer of tumor–cord interface. At the final stage of tumor resection, draining veins were coagulated and sharply cut. Complete resection of the tumor was performed under a microscope and confirmed using ICG-VA [Figure 3f].
Figure 3.
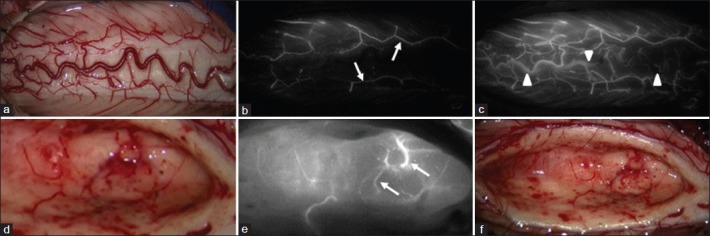
Intraoperative photographs from Patient 4. (a) Photographs showing a slightly swollen spinal cord. (b) Photographs from the early phase of indocyanine green videoangiography (ICG-VA) showing the feeding arteries (arrows), tumor and draining veins (arrowheads). (c) Photographs showing a decrease in blood flow to the tumor after interruption of feeding arteries using small aneurysm clips. (d) Photographs showing the clear gliosis layer of tumor–cord interface after careful dissection of thick and cloudy pia mater that encircled the tumor. (e-f) ICG-VA images obtained after en bloc complete removal of the hemangioblastoma showing no sign of residual tumor and well preserved posterior spinal artery
The pial edges were sutured together as much as possible to restore the shape of the spinal cord.[1,5,16,21] The arachnoid membrane was then closed with the dura mater or autologous graft of fascia lata to reduce the chance of postoperative arachnoid adhesion. Resected laminae were constructed as a lift-up style (for the cervical spine) or an on-lay style (for the thoracic spine) using a titanium mini-plate and screws.[4,19]
RESULTS
Twenty-seven injections of ICG-VA were performed in 14 cases. Characteristics of patients and tumors studied with ICG-VA are summarized in Table 1. There were no complications or side-effects related to ICG-VA. The flow dynamics of spinal microvasculature, including the tumor itself, were well visualized within 10 seconds of intravenous systemic injection of ICG. ICG angiographic images could be divided into arterial, capillary, and venous phases, and the image resolution was in high quality, even for small perforators that are usually almost impossible to see using standard intraoperative digital subtraction angiography. Intraoperative ICG-VA provided dynamic flow images of the spinal microvasculature in accordance with the progress of surgical procedures. All angiographic images were well integrated into the microscopic view. It took less than 5 min in all cases from the start one systemic injection until the interpretation of the angiographic images.
The utility of ICG-VA could be summarized into three categories: (1) Localization of normal spinal arteries and veins, (2) assessment of posterior spinal venous circulation, and (3) differentiation of feeding arteries, tumor, and draining veins. Posterior spinal arteries were well differentiated from posterior spinal veins and the pial venous plexus. The assessment of venous circulation of posterior spinal veins and the pial venous plexus crossing the PMS or PLS was easy. At the final stage of the surgical procedure, spinal microvasculature, such as posterior sulcal central veins or sulcal central branches of the anterior spinal artery, was well analyzed. Typically, feeding arteries to hemangioblastomas were well differentiated from draining veins and were interrupted just proximal to the hemangioblastoma. After the interruption of feeding arteries, the partial or complete decrease in blood flow to the tumor was evident on ICG-VA.
Illustrative case 1
Patient 3: A 42-year-old female was admitted with back pain, gait disturbance, and moderate dysesthesia of right upper extremity. Symptoms were more apparent in the upper extremities than in the lower extremities. Assessment of neurological condition before surgery suggested Grade 3 on the modified McCormick functional schema and Grade 3 on the sensory pain scale. T2-weighted magnetic resonance images (MRIs) of the cervical spine showed local enlargement and intramedullary mixed signal of the spinal cord at C6 [Figure 4a]. T2*-weighted gradient-echo MRIs showed mixed low signal within the spinal cord [Figure 4b], which was consistent with blood degradation products. Imaging diagnosis before surgery was intramedullary hemorrhage associated with cavernous malformation.
Figure 4.
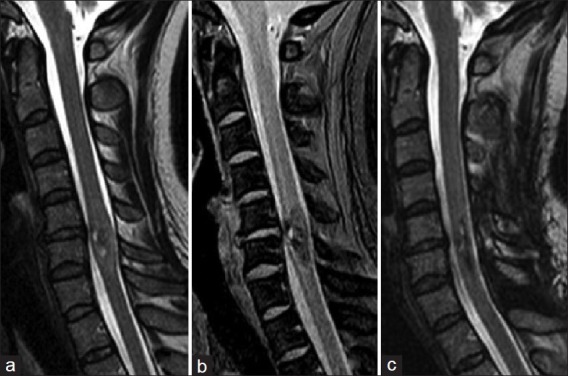
Patient 3.(a) Preoperative T2-weighted MR images showing local enlargement and intramedullary mixed signal of the spinal cord at C6. (b) Preoperative T2*-weighted gradient-echo MR images showing mixed low signal within the spinal cord, suggesting the deposition of blood degradation products. (c) Postoperative T2-weighted MR images showing satisfactory recovery of intramedullary signal of the spinal cord
The patient underwent C5 to C7 osteoplastic laminotomy for evacuation of the intramedullary hemorrhage and total resection of the tumor. The spinal cord appeared slightly swollen [Figure 1a]. Posterior spinal arteries on both sides were well differentiated from posterior spinal veins and the pial venous plexus [Figure 1b]. Venous circulation of posterior spinal veins and the pial venous plexus appeared stagnant [Figure 1c]. Crossing veins on the PMS were coagulated with a microbipolar coagulator at very low power levels under continuous saline irrigation. A PMS approach to the spinal cord revealed the intramedullary hemorrhage. Careful dissection within the spinal cord revealed the tumor–cord interface with the surrounding hemosiderin-stained tissue. The tumor was removed segmentally. The surrounding hemosiderin-stained tissue was not resected to avoid the damage of spinal tissue [Figure 1d]. Posterior sulcal central veins were well visualized [Figure 1e]. The pial edges were sutured together as much as possible to restore the shape of the spinal cord [Figure 1f]. Supplemental digital content of surgical video Patient 3 was provided.
T2-weighted MRIs obtained 3 months after surgery showed satisfactory recovery of the intramedullary signal of the spinal cord [Figure 4c]. Assessment of neurological condition at 3 months after surgery suggested Grade 2 on the modified McCormick functional schema and Grade 2 on the sensory pain scale.
Illustrative case 2
Patient 12: A 46-year-old female was admitted with severe dysesthetic pain radiating from the posterior neck to the right upper extremity. Assessment of neurological condition before surgery suggested Grade 2 on the modified McCormick functional schema and Grade 3 on the sensory pain scale. T2-weighted MRIs of the cervical spine showed an intramedullary tumor with syrinx formation at C6 [Figure 5a]. The tumor was well enhanced on T1-weighted images [Figure 5b]. Imaging diagnosis before surgery was intramedullary ependymoma.
Figure 5.

Patient 12. (a) Preoperative T2-weighted MR images showing intramedullary tumor formation at C6/7 accompanying moderate syrinx. (b) Preoperative contrast-enhanced T1-weighted MR images showing the enhanced tumor within the spinal cord. (c) Postoperative T2-weighted MR images showing satisfactory recovery of intramedullary signal of the spinal cord
The patient underwent C5 to C7 osteoplastic laminotomy for evacuation of the intramedullary hemorrhage and total resection of the tumor. The spinal cord appeared slightly swollen [Figure 2a]. Posterior spinal arteries on both sides were well differentiated from posterior spinal veins and the pial venous plexus [Figure 2b]. Venous circulation of posterior spinal veins and the pial venous plexus was stagnant [Figure 2c]. Crossing veins on the PMS were coagulated with a microbipolar coagulator at very low power levels under continuous saline irrigation. A PMS approach to the spinal cord revealed the tumor surface. Careful dissection within the spinal cord revealed the tumor–cord interface with the surrounding gliotic tissue. The tumor was removed in an en bloc fashion. Sulcal central branches of the anterior spinal artery were well preserved [Figure 2d and e]. The surrounding gliotic tissue was not resected to avoid the damage of spinal tissue [Figure 2f]. The pial edges were sutured together as much as possible to restore the shape of the spinal cord.
T2-weighted MRIs obtained 3 months after surgery showed satisfactory recovery of the intramedullary signal of the spinal cord [Figure 5c]. Assessment of neurological condition at 3 months after surgery suggested Grade 2 on the modified McCormick functional schema and Grade 2 on the sensory pain scale.
Illustrative case 3
Patient 4: A 17-year-old male was admitted with moderate dysesthetic pain radiating from the posterior neck to the right upper extremity. Assessment of neurological condition before surgery suggested Grade 1 on the modified McCormick functional schema and Grade 3 on the sensory pain scale. T2-weighted MRIs of the cervical spine showed an intramedullary tumor formation at C6/7 accompanying extensive syrinx [Figure 6a]. The tumor was enhanced on T1-weighted MRIs [Figure 6b]. Imaging diagnosis before surgery was intramedullary hemangioblastoma.
Figure 6.
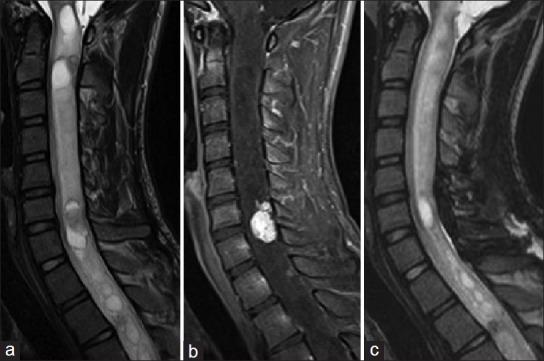
Patient 4. (a) Preoperative T2-weighted MR images showing intramedullary tumor formation at C6/7 accompanying extensive syrinx. (b) Preoperative contrast-enhanced T1-weighted MR images showing the enhanced tumor within the spinal cord. (c) Postoperative T2-weighted MR images showing complete resection of the tumor
The patient underwent C5 to C7 osteoplastic laminotomy for total resection of the tumor. The spinal cord appeared slightly swollen [Figure 3a]. Feeding arteries to the tumor were well differentiated from draining veins [Figure 3b], and were intercepted just proximal to the tumor. Blood flow to the tumor decreased during occlusion of feeding arteries [Figure 3c]. Thick and cloudy pia mater encircling the tumor was cut sharply to reveal the clear gliosis layer of tumor–cord interface [Figure 3d]. The tumor was completely removed in an en bloc fashion under the microscope [Figure 3e]. When tumor removal was complete, there was little or no need for further hemostasis. ICG-VA after en bloc complete removal of the tumor revealed no sign of residual tumor and well preserved posterior spinal artery [Figure 3f]. The pial edges were sutured together as much as possible to restore the shape of the spinal cord. Supplemental digital content of surgical video Patient 4 was provided.
T2-weighted MRIs obtained 3 months after surgery showed complete resection of the tumor [Figure 6c]. Assessment of neurological condition at 3 months after surgery suggested Grade 1 on the modified McCormick functional schema and Grade 1 on the sensory pain scale.
DISCUSSION
The present study showed that intraoperative vascular flow assessment using ICG-VA was easy, repeatable, and practical without any significant procedure-related risks. ICG angiographic images could be divided into arterial, capillary, and venous phases, and the image resolution was in high quality, even for small perforators that are usually almost impossible to see using standard intraoperative digital subtraction angiography. Intraoperative ICG-VA provided dynamic flow images of the spinal microvasculature in accordance with the progress of surgical procedures. All angiographic images were well integrated into the microscopic view. The utility of ICG-VA could be summarized into three categories: (1) Localization of normal spinal arteries and veins, (2) assessment of posterior spinal venous circulation, and (3) differentiation of feeding arteries, tumor, and draining veins.
The first successful removal of a spinal intramedullary tumor was performed by Von Eiselberg in 1907,[14] and Elsberg and Beer reported the operability of intramedullary tumors of the spinal cord in 1911.[2] These pioneering achievements have been handed down to establish the basic concept and strategy for the surgical management of spinal intramedullary tumors, and many other reports were followed.[1,2,3,6,7,9,11,14,15,16,22] However, spinal intramedullary tumors are still the major challenge for neurosurgeons, particularly considering their infrequency and unknown natural history of spinal intramedullary tumors, and the difficulty in standardizing treatment. Developments in microsurgical instruments, medical equipment, and neurophysiological monitoring have increased the safety and precision of surgery, and intraoperative image guidance using near-infrared ICG-VA is now available to provide a real-time angiographic images during surgery.[8,10,12,13,17,18,20] The present study focused the analysis of the flow dynamics of spinal microvasculature using ICG-VA to achieve an additional level of safety as well as precision with the surgical procedure. ICG-VA improves vascular flow orientation during surgery, and is particularly useful in assessing cerebrovascular aneurysms, vascular bypass surgery, or arteriovenous malformations of the brain or spinal cord. The limited field-of-view is the main disadvantage of ICG-VA.
Microvascular flow or anatomical orientation of the spinal cord needs to be ensured for safe and precise surgery. The vascular pattern on the dorsal spinal cord usually appeared abnormal, although not too formidable. Differentiation of the posterior spinal artery and its branches from the posterior spinal veins and the venous plexus is not always easy, and vascular flow of the posterior spinal veins or the venous plexus is not fully assessed. The present study suggested that localization of normal spinal arteries and veins as well as assessment of posterior spinal venous circulation is possible with the help of ICG-VA. An additional difficulty sometimes lay in the vascular pedicles that supply the tumor and are connected to the anterior spinal artery. Careful coagulation and division of the small arterial feeders to the tumor resulted in safe and precise resection of the tumor. Spinal hemangioblastomas are mostly superficial, lying over and into the spinal cord, and are highly vascular tumors that require advanced surgical techniques to achieve safe and precise resection. A critical point for safe resection of spinal hemangioblastomas is preservation of the main draining veins until the feeding arteries are well controlled. In particular, for huge hemangioblastomas, the draining vein should not be coagulated at the beginning of the procedure. A thorough inspection of the feeding arteries under a microscope is possible with the help of ICG-VA, and the effect of interruption of the feeding arteries on blood flow to the tumor can be also assessed. After en bloc resection of the tumor, vascular flow assessment of normal spinal cord can be finally confirmed on ICG-VA. The results of this study indicate that ICG-VA can help surgeons to assess the dynamic flow images of the spinal microvasculature in accordance with the progress of surgical procedures. Although ICG-VA may offer valuable information for surgeons, surgeons should be not only be well versed in regional anatomy, but also attach importance to meticulous nonbleeding procedure.
CONCLUSIONS
The present study focused the analysis of the flow dynamics of spinal microvasculature using ICG-VA to achieve an additional level of safety as well as precision with the surgical procedure. Intraoperative vascular flow assessment using ICG-VA was easy, repeatable, and practical without any significant procedure-related risks. ICG-VA can be used for careful analysis of spinal microvascular flow or anatomical orientation, which is necessary to ensure safe and precise resection of spinal intramedullary tumors.
Videos available at www.surgicalneurologyint.com
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2013/4/1/135/119352
Disclaimer: The authors of this article have no conflicts of interest to disclose, and have adhered to SNI's policies regarding human/animal rights, and informed consent. Advertisers in SNI did not ask for, nor did they receive access to this article prior to publication.
Contributor Information
Toshihiro Takami, Email: ttakami@med.osaka-cu.ac.jp.
Toru Yamagata, Email: tyamagata@med.osaka-cu.ac.jp.
Kentaro Naito, Email: 7110ken622@med.osaka-cu.ac.jp.
Hironori Arima, Email: a03ma002@yahoo.co.jp.
Kenji Ohata, Email: kohata@med.osaka-cu.ac.jp.
REFERENCES
- 1.Brotchi J. Intrinsic spinal cord tumor resection. Neurosurgery. 2002;50:1059–63. doi: 10.1097/00006123-200205000-00021. [DOI] [PubMed] [Google Scholar]
- 2.Elsberg CA, Beer E. The operability of intramedullary tumors of the spinal cord. A report of two operations, with remarks upon the extrusion of intraspinal tumors. Am J Med Sci. 1911;142:636–47. [Google Scholar]
- 3.Garrido E, Stein BM. Microsurgical removal of intramedullary spinal cord tumors. Surg Neurol. 1977;7:215–9. [PubMed] [Google Scholar]
- 4.Goto T, Ohata K, Takami T, Nishikawa M, Tsuyuguchi N, Morino M, et al. Hydroxyapatite laminar spacers and titanium miniplates in cervical laminoplasty. J Neurosurg. 2002;97:323–9. doi: 10.3171/spi.2002.97.3.0323. [DOI] [PubMed] [Google Scholar]
- 5.Goto T, Ohata K, Takami T, Nishikawa M, Nishio A, Morino M, et al. Prevention of postoperative posterior tethering of spinal cord after resection of ependymomas. J Neurosurg. 2003;99:181–7. doi: 10.3171/spi.2003.99.2.0181. [DOI] [PubMed] [Google Scholar]
- 6.Greenwood J., Jr Total removal of intramedullary tumors. J Neurosurg. 1954;11:616–21. doi: 10.3171/jns.1954.11.6.0616. [DOI] [PubMed] [Google Scholar]
- 7.Guidetti B. Intramedullary tumours of the spinal cord. Acta Neurochir. 1967;17:7–23. doi: 10.1007/BF01670413. [DOI] [PubMed] [Google Scholar]
- 8.Hänggi D, Etminan N, Steiger HJ. The impact of microscope-integrated intraoperative near-infrared indocyanine green videoangiography on surgery of arteriovenous malformations and duralarteriovenous fistulae. Neurosurgery. 2010;67:1094–104. doi: 10.1227/NEU.0b013e3181eb5049. [DOI] [PubMed] [Google Scholar]
- 9.Hoshimaru M, Koyama T, Hashimoto N, Kikuchi H. Results of microsurgical treatment for intramedullary spinal cord ependymomas: Analysis of 36 cases. Neurosurgery. 1999;44:264–9. doi: 10.1097/00006123-199902000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Hwang SW, Malek AM, Schapiro R, Wu JK. Intraoperative use of indocyanine green fluorescence videography for resection of a spinal cord hemangioblastoma. Neurosurgery. 2010;67:ons 300–3. doi: 10.1227/01.NEU.0000383876.72704.7B. [DOI] [PubMed] [Google Scholar]
- 11.Iwasaki Y, Hida K, Sawamura Y, Abe H. Spinal intramedullary ependymomas: Surgical results and immunohistochemical analysis of tumour proliferation activity. Br J Neurosurg. 2000;14:331–6. doi: 10.1080/026886900417315. [DOI] [PubMed] [Google Scholar]
- 12.Killory BD, Nakaji P, Gonzales LF, Ponce FA, Wait SD, Spetzler RF. Prospective evaluation of surgical microscope-integrated intraoperative near-infrared indocyanine green angiography during cerebral arteriovenous malformation surgery. Neurosurgery. 2009;65:456–62. doi: 10.1227/01.NEU.0000346649.48114.3A. [DOI] [PubMed] [Google Scholar]
- 13.Kim EH, Cho JM, Chang JH, Kim SH, Lee KS. Application of intraoperative indocyanine green videoangiography to brain tumor surgery. Acta Neurochir (Wien) 2011;153:1487–95. doi: 10.1007/s00701-011-1046-x. [DOI] [PubMed] [Google Scholar]
- 14.Malis LI. Intramedullary spinal cord tumors. Clin Neurosurg. 1978;25:512–39. doi: 10.1093/neurosurgery/25.cn_suppl_1.512. [DOI] [PubMed] [Google Scholar]
- 15.McCormick PC, Torres R, Post KD, Stein BM. Intramedullary ependymoma of the spinal cord. J Neurosurg. 1990;72:523–32. doi: 10.3171/jns.1990.72.4.0523. [DOI] [PubMed] [Google Scholar]
- 16.Ohata K, Takami T, Gotou T, El-Bahy K, Morino M, Maeda M, et al. Surgical outcome of intramedullary spinal cord ependymoma. Acta Neurochir. 1999;141:341–7. doi: 10.1007/s007010050309. [DOI] [PubMed] [Google Scholar]
- 17.Raabe A, Beck J, Gerlach R, Zimmermann M, Seifert V. Near-infrared indocyanine green video angiography: A new method for intraoperative assessment of vascular flow. Neurosurgery. 2003;52:132–9. doi: 10.1097/00006123-200301000-00017. [DOI] [PubMed] [Google Scholar]
- 18.Rodríguez-Hernández A, Lawton MT. Flash fluorescence with indocyanine green videoangiography to identify the recipient artery for bypass with distal middle cerebral artery aneurysms: Operative technique. Neurosurgery. 2012;70:209–20. doi: 10.1227/NEU.0b013e31823158f3. [DOI] [PubMed] [Google Scholar]
- 19.Takami T, Ohata K, Goto T, Nishikawa M, Nishio A, Tsuyuguchi N, et al. Lift-up laminoplasty for myelopathy caused by ossification of the posterior longitudinal ligament of the cervical spine. Neurol India. 2004;52:59–63. [PubMed] [Google Scholar]
- 20.Takami T, Yamagata T, Mitsuhashi Y, Hayasaki K, Ohata K. Direct surgery for spinal arteriovenous fistulas of the filumterminale with intraoperative image guidance. Spine. 2012;37:E1524–8. doi: 10.1097/BRS.0b013e31826f20c0. [DOI] [PubMed] [Google Scholar]
- 21.Takami T, Yamagata T, Ohata K. Posterolateral sulcus approach for spinal intramedullary tumor of lateral location. Technical Note. Neurol Med Chir. doi: 10.2176/nmc.tn2012-0419. [In Press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yasargil MG, Antic J, Laciga R, de Preux J, Fideler RW, Boone SC. The microsurgical removal of intramedullary spinal hemangioblastomas. Report of twelve cases and a review of the literature. Surg Neurol. 1976;3:141–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


