Abstract
Background
The HIV-2 env’s 3’ end encodes the cytoplasmic tail (CT) of the Env protein. This genomic region also encodes the rev, Tat and Nef protein in overlapping reading frames. We studied the variability in the CT coding region in 46 clinical specimens and in 2 reference strains by sequencing and by culturing. The aims were to analyse the variability of Env CT and the evolution of proteins expressed from overlapping coding sequences.
Results
A 70% reduction of the length of the CT region affected the HIV-2 ROD and EHO strains in vitro due to a premature stop codon in the env gene. In clinical samples this wasn’t observed, but the CT length varied due to insertions and deletions. We noted 3 conserved and 3 variable regions in the CT. The conserved regions were those containing residues involved in Env endocytosis, the potential HIV-2 CT region implicated in the NF-kB activation and the potential end of the lentiviral lytic peptide one. The variable regions were the potential HIV-2 Kennedy region, the potential lentiviral lytic peptide two and the beginning of the potential lentiviral lytic peptide one. A very hydrophobic region was coded downstream of the premature stop codon observed in vitro, suggesting a membrane spanning region. Interestingly, the nucleotides that are responsible for the variability of the CT don’t impact rev and Nef. However, in the Kennedy-like coding region variability resulted only from nucleotide changes that impacted Env and Tat together.
Conclusion
The HIV-2 Env, Tat and Rev C-terminal part are subject to major length variations in both clinical samples and cultured strains. The HIV-2 Env CT contains variable and conserved regions. These regions don’t affect the rev and Nef amino acids composition which evolves independently. In contrast, Tat co-evolves with the Env CT.
Introduction
The human immunodeficiency virus type 2 (HIV-2) env gene encodes the envelope polyglycoprotein (Env) that is cleaved inside the cell by an endogenous protease and leads to the production of two glycoproteins (gpSU and gpTM) [1,2].
gpSU is present at the surface of the envelope while gpTM is a transmembrane glycoprotein. The gpTM contains four major parts: the fusion peptide and the heptad repeats which are located outside the virus [3-5], the transmembrane region [6], and the C-terminal domain which is the only internal region of Env and is called the cytoplasmic tail (CT).
Little data is known about the HIV-2 CT, but the HIV-1 CT contains subregions namely, from its N-terminal to C-terminal part, the endocytosis signal sequence, the Kennedy sequence, three lentiviral lytic peptides (LLP) and a final di-Leucine motif [7-18]. The latter is also involved in the process of Env endocytosis [10,11]. The Kennedy sequence contains epitopes that are recognised by antibodies when they are expressed in rabbits [12-14]. Finally, the three HIV-1 LLPs are regions that can alter the permeability of the cell membrane [15-18]. Except for the identification of the endocytosis signal, no systematic comparison of the patterns of the HIV-1 and HIV-2 CT has been published to date [19].
The HIV-2 env gene contains the nucleotide sequences that encode Tat, Rev and the N-terminal part of Nef in overlapping reading frames. The 3’end of the env gene that expresses HIV-2 CT is the region where the overlap is the most important as 4 proteins are expressed from that sequence. The study of the 3’ end of the env gene constitutes an interesting model for the characterisation of the poorly known HIV-2 CT and for a study of the evolution of proteins expressed from different reading frames in a single sequence.
For this purpose, we sequenced the CT coding region from in vitro adapted strains and from clinical samples at different stages of the disease. The env coding sequences obtained were then used to analyse the CT variability, and to study the impact of the CT variability on the other proteins expressed from the same nucleotides sequence.
Results
HIV-2 Env CT full-length is not mandatory in vitro
We sequenced the reference strains ROD and EHO (representing respectively HIV-2 groups A and B) after several passages in vitro in H9 cells. We found several differences when we compared the sequences of our cultured virus with the published reference sequences: ROD (Genbank:M15390) and EHO (Genbank:U27200), (Table 1). The most important difference was the replacement of a tryptophan codon (TGG) by a stop codon at the 748th EHO env codon and 750th ROD env codon (always TAG). We also confirmed this adaption of the CT length with three independent experiments in which the infectious clone pKP59-ROD, initially cloned from a clinical sample [20], was transfected in 293 cells and passaged several times on MT2, MT4 and H9 cells. This phenomenon was thus constant in various lymphoid lineages and was not strain-specific.
Table 1. HIV-2 ROD and EHO reference strain aa sequence alteration after H9 cell line passages.
|
HIV-2 ROD (group A)
|
HIV-2 EHO (group B)
|
||||
|---|---|---|---|---|---|
| Env Aa position | Aa in the reference | Aa in the in vitro selected virus | Env Aa position | Aa in the reference | Aa in the in vitro selected virus |
| 727 | R | R/Q | 700 | R | R/Q |
| 728 | G | E | 720 | P | P/L |
| 749 | P | L | 723 | K | K/E |
| 750 | W | STOP | 724 | D | D/E |
| 792 | R | R/K | 725 | R | R/W |
| 793 | D | D/N | 739 | N | N/D |
| 794 | W | W/STOP | 740 | N | N/D |
| 796 | R | R/K | 741 | R | R/G |
| 798 | R | R/K | 747 | P | P/L |
| 800 | A | A/T | 748 | W | STOP |
| 815 | A | T | 756 | P | L |
| 821 | R | R/K | 757 | I | I/F |
| 840 | R | K | 761 | R | R/K |
| 841 | G | G/E | 788 | P | P/L |
| 848 | R | R/K | 793 | P | L |
In the table, we can observe: the Env position of the substitution, the aa substituted and the new aa (X/Y corresponding to a mixture of aa). CT starts at 701st Env position for ROD and 699th for EHO.
The entire HIV-2 CT length is required in vivo
We sequenced the 46 CT coding region obtained from 27 different patients at various clinical stages (Table 2). Thirty of those 46 sequences were obtained from plasma RNA and 16 from proviral DNA samples. Although we observed that the CT length varied from 148 to 165 amino acids (aa) due to insertions and deletions in the DNA/RNA sequences, we did not find any premature stop codon. In contrast to what was observed in vitro, a full-length CT was found in all clinical samples.
Table 2. Patient cohort description (N=27).
| Genbank accession number | Plasma viral load (copies/mL) | CD4 count (cell/mL) | AIDS (CDC definition) | ARV before sampling | Gender | Age at sampling |
|---|---|---|---|---|---|---|
| KC748535 | 6450 | 166 | Y | Y | M | 56 |
| KC748536 | 187000 | 227 | N | Y | F | 54 |
| KC748538 | 3155 | 310 | N | Y | M | 53 |
| KC748540 | 1420 | 330 | N | Y | M | 53 |
| KC748544 | 1495 | 500 | N | Y | M | 48 |
| KC748547 | 575 | 775 | N | N | M | 48 |
| KC748548 | 565 | 287 | N | N | M | 44 |
| KC748549 | 1065 | 434 | N | N | M | 44 |
| KC748550 | 35750 | 266 | N | Y | M | 46 |
| KC748551 | 2025 | 233 | N | N | M | 43 |
| KC748552 | 1150 | 451 | N | N | F | 43 |
| KC748553 | 555 | 219 | N | N | M | 41 |
| KC748555 | 47435 | N.A. | N | N | F | 40 |
| KC748556 | 405 | 581 | Y | N | F | 39 |
| KC748557 | 1770 | 718 | N | N | F | 34 |
| KC748558 | 435 | N.A. | N | N | F | 32 |
| KC748559 | 480 | N.A. | N | N | M | 32 |
| KC748561 | 4165 | N.A. | N | N | F | 28 |
| KC748562 | 735 | N.A. | N | N | M | 21 |
| KC748563 | 14800 | N.A. | N | Y | M | 10 |
| KC748564 | 515000 | 100 | Y | Y | M | 33 |
| KC748566 | 185 | 704 | N | N | M | 51 |
| KC748567 | 5250 | 162 | N | N | F | 50 |
| KC748577 | <50 | 531 | N | Y | F | 47 |
| KC748578 | <50 | 492 | Y | Y | F | 42 |
| KC748579 | 74 | N.A. | N | N | F | 35 |
| KC748580 | <50 | 356 | Y | Y | M | 50 |
For each patient we show: accession number of the sequences identical to Figure 1, viral load, CD4 count, presence of AIDS (Yes=Y, No=N), antiretroviral (ARV) treatment before sampling, gender (Male=M, Female= F) and age at sampling. N.A.= Not available.
Analysis of the sequences
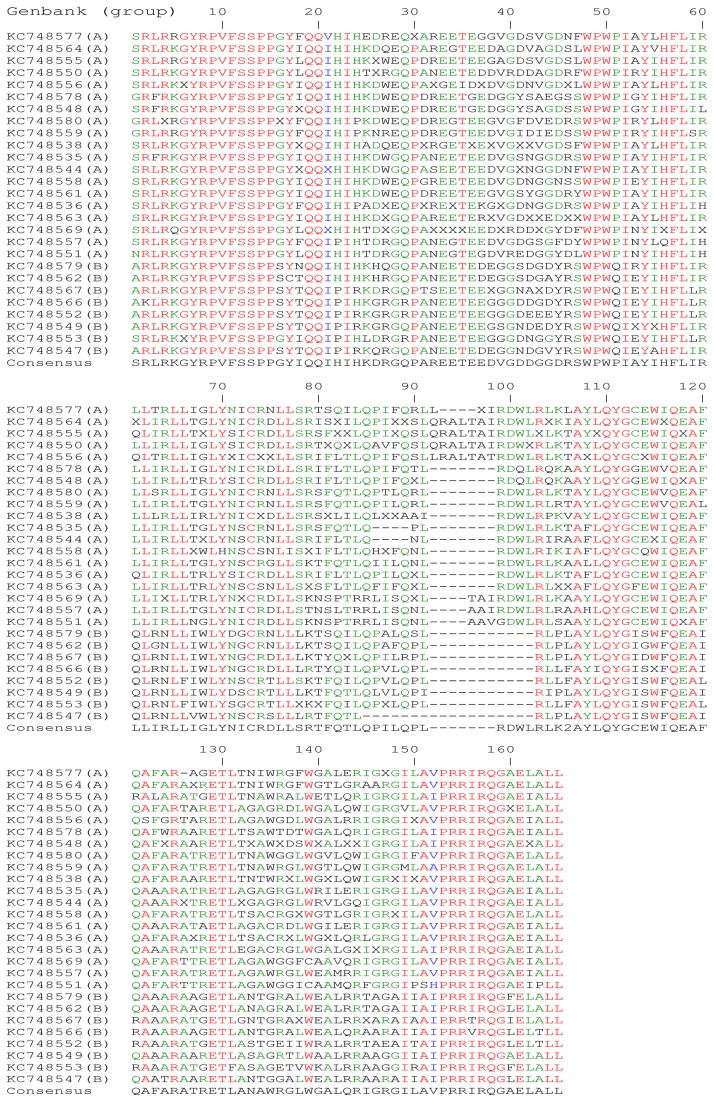
The region studied by sequencing started from the env codon 701 for HIV-2 ROD and from env codon 698 for EHO up to the env stop codon. This region is homologous to the CT coding region of HIV-1. Figure 1 shows the alignment of 27 aa sequences including one sequence per patient. That alignment was used to study the Shannon Entropy (SE) of each aa position as well as the frequency of positions with a mixtures of aa along the CT region (Table 3). The SE can be seen as a measure of the variability of each position in the sequences [21]. The 165 positions of the alignment were divided into 7 regions, named A to G in Table 3. The CT started either with a serine, a glycine or an alanine. In all HIV-2 group A sequences a serine start was predominant and an alanine start was dominant in all HIV-2 group B sequences. All of our sequences ended with a leucine.
Figure 1. Alignment of 27 virus/provirus CT HIV-2 aa sequences (1 sequence per patient), classified by similarity.
The GenBank accession number is followed by the HIV-2 group (A or B). To construct the alignment, only the most recent sample was included. The conserved aa are highlighted in red (>85% of conservation). In green appears the most represented aa for each position (>50% of conservation). Blue corresponds to variable positions with similar aa.
Table 3. Study of the variability and hydrophobicity of the different CT regions of HIV-2.
| Region (CT aa positions) | Average SE | Average mixture of aa per position | Kyte-Doolittle scale score average | Average variation per position per alignment** | Average SE provius alignment/ virus alignment* |
|---|---|---|---|---|---|
| CT (1 to 165) | 0.618 | 0.570 | -0.295 | 0.511 | 0.229/0.200 |
| A (0 to 20) | 0.253 | 0.15 | -0.616 | 0.250 | 0.121/0.068 |
| B (21 to 47) | 0.898 | 1.11 | -1.813 | 0.889 | 0.360/0.283 |
| B group A* | 0.278 | ND | ND | ND | ND |
| B group B* | 0.193 | ND | ND | ND | ND |
| C (48 to 68) | 0.504 | 0.428 | 1.190 | 0.333 | 0.192/0.152 |
| D (69 to 91) | 0.807 | 0.957 | 0.023 | 0.479 | 0.232/0.252 |
| E (92 to 125) | 0.565 | 0.324 | -0.195 | 0.441 | 0.189/0.208 |
| F (126 to 136) | 0.691 | 0.364 | -0.495 | 0.272 | 0.292/0.216 |
| G (137 to 165) | 0.592 | 0.517 | -0.032 | 0.724 | 0.228/0.191 |
| G start (137 to 150) | 0.968 | 1.071 | 0.216 | 1.286 | 0.360/0.312 |
| G end (151 to 165) | 0.242 | 0.067 | -0.382 | 0.200 | 0.105/0.079 |
A: the first conserved region containing the endocytosis signal. B: the region containing the Kennedy-like sequences. C: the hydrophobic region D: LLP-2 domain. E: LLP-3 domain. F: LLP-3 / LLP-1 inter-region. G: LLP-1 region. ND: not determined. * Group A, B, provirus, virus alignement SE were normalized with the natural logarithm of the number of sequences. ** Results of the intra-patient study.
The first 20 CT aa positions were highly conserved (consensus SRLRKGYRPVFSSPPGYIQQ; region A). In this region, the conserved motif GYRPV corresponded to the endocytosis signal (position 6 to 10). A second well-conserved sequence extended from positions 13 to 20, with a consensus SSPPG/SYXQQ, where the X was a variable aa, G being present for all group A sequences and S for all group B sequences. The latter was homologous to a motif that was recently described and which interacts with NF-kB expression in SIVmac [22].
The most variable region of the full sequence extended from residues 21 to 47 with a consensus IHIHKDRGQPAREETEEDVGDDGGDRS in region B shown in Table 3. This region contains also the highest mixture of aa (Table 3). As this region contained aa homologous to the HIV-1 Kennedy sequence, we named it the Kennedy-like region.
The 22 aa that follow this variable region (position 48 to 69, consensus WPWPIAYIHFLIRLLIRLLIGL; region B) were much more conserved, especially the two double leucine motifs coloured in italic (region C, Table 3). This region is also the most hydrophobic of the entire CT as shown by the Kyte-Doolittle scale score average (Table 3). The sequence of this CT region was interrupted after the first proline in the cultured virus, where a stop codon replaced the codon of the second tryptophan.
The last 96 CT positions contained residues homologous to the three HIV-1 LLPs. The first part should contain a region homologous to the LLP-2 (position 70 to 91 consensus YNICRDLLSRTFQTLQPILQPL; region D). This region was not conserved among our sequences and between the HIV-2 consensus and HIV-1 sequences, except for a punctual di-leucine motif and a tyrosine (in italic). Moreover, this region contained mixture of aa and we noted that some viruses had a deletion at the last position of this region (Figure 1).
The next region, homologous to the HIV-1 LLP-3 region, spanned from positions 92 to 125 (consensus -------RDWLRLKXAYLQYGCEWIQEAFQAFA, with a dash corresponding to the absence of aa as consensus; region E). The putative LLP-3 started by a complex situation with some sequences containing insertions or deletions in comparison with the consensus. Despite this complex situation, the rest of the sequences were not variable.
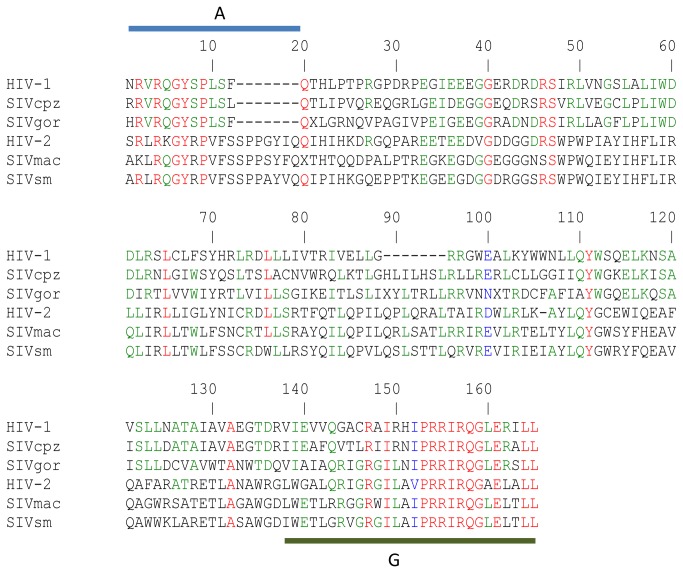
We divided the two final regions of our sequences in two arginine rich regions. The first region was a small LLP-3/LLP-1 inter-region (position 126 to 136 consensus RATRETLANAWR; region F, Table 3), and the second was the LLP-1 homologous sequence (position 137 to 165 consensuses GLWGALQRIGRGIL/AVPRRIRQGAELALL; with the final di-leucine motif; region G). The beginning of the LLP-1 region was highly variable (position 137 to 150, aa before the slash; G start, Table 3). This part contained also a lot of positions with mixtures of aa. In contrast, the end of the LLP-1 (aa after the slash) was the most constant part of the CT (G end, Table 3). Interestingly, this LLP-1 region was very rich in arginine and ended in all our sequences with the final di-leucine motif. Furthermore, the LLP-1 conserved part is also conserved among all primate lentiviruses (Figure 2).
Figure 2. Env CT sequence alignment of the major primate lentiviruses.
HIV-1: (Swiss-prot: P04578), SIVcpz: (Swiss-prot: P17281), SIVgor: (GenBank:FJ424865.1), HIV-2: CT consensus sequence from the article, SIVmac: (Swiss-prot: P08810) and SIVsm: (Swiss-prot: P12492). The conserved aa (in red) are aa of the endocytosis signal (region A) and the aa at the end of the LLP-1 region (region G). The aa implicated in SIVmac NF-kB activation (end of region A) are absent in HIV-1 and its lineage (SIVcpz and gor).
HIV-2 CT sequences variability over time in infected individuals
The sequence evolution over time was studied in eight patients from whom we had more than one HIV-2 CT sequence available, which could be either exclusively viral or exclusively proviral. Nine alignments were performed with 25 sequences (1 alignment per patient except 1 patient with two alignments). As the number of sequences per alignment was limited, the use of the SE for the analysis of the variability was not suitable. Therefore, we measured the number of times where a CT position varied in each of the intra-patient alignments (Table 3). We observed that 40% of the positions varied over time in contrast to 76% of variable positions in the inter-patient alignment. The variable and constant regions were similar between the intra-patient and the inter-patient study. However, the regions D and F were less variable in the intra-patient study compared to the inter-patient evaluation (Table 3).
Provirus-virus sequences comparison
We compared provirus and virus sequences from 4 patients for whom the sequences for both the provirus and the virus were available. We observed similar differences compared to the previous intra-patient study virus, but in one case a small insertion was present in the provirus and absent from the plasma RNA. Notably, this insertion was located in the LLP-3 complex coding region: virus (Genbank: KC748547) and provirus (Genbank: KC748572). As the number of patients was not sufficient to conclude that CT variability from RNA virus samples and DNA provirus is similar, we made two alignments with only one provirus or one virus sample per patient, resulting in 10 proviral DNA sequences (6 from group A) and 21 viral sequences (15 from group A), respectively. We measured the normalised SE from those alignments, by the natural log of the number of sequences (Table 3). The mean of the normalised SE was slightly more important in proviral DNA, possibly due to different ratios in group A and B sequences. However, the variable and constant regions were similar between provirus and virus sequence alignments. Therefore the choice of provirus or virus sequences did not affect the definition of variable and constant regions.
Influence of Tat, Rev and Nef on HIV-2 CT variability
The sequences of the env gene that we analysed are shared with the 2nd exon of tat and rev and with the first half of nef. We analysed the reciprocal impact of Tat, Rev and Nef variability on the HIV-2 CT regions.
The 2nd exon of tat Open Reading Frame (ORF) starts at the 16th /17th codon of the CT, with the first nucleotides of each tat codons corresponding to the 2nd nucleotide of each env codons. The beginning of the 2nd tat exon is followed, 5 env codons later, by the beginning of the 2nd exon of rev, with the first nucleotides of each rev codons corresponding to the 3rd nucleotide of each env codon. Nef starts to be expressed at the 110th /111th CT codon, and is translated in the same reading frame as tat.
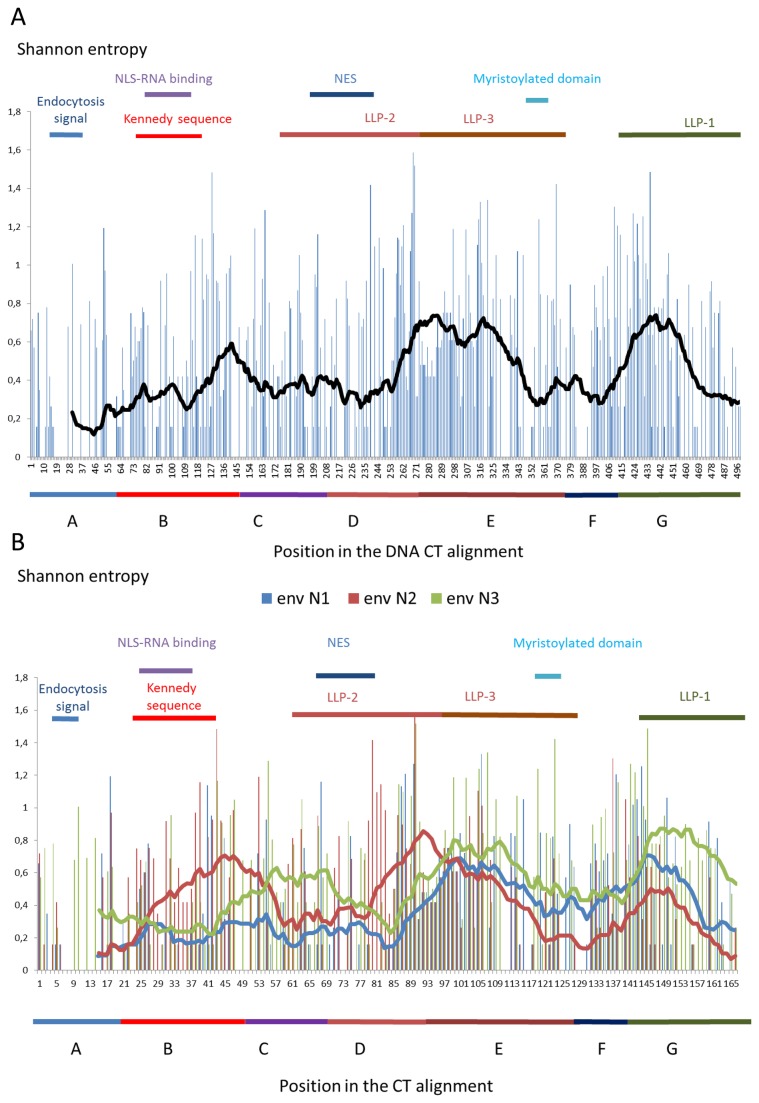
Figures 3a and 3b show the SE of the entire CT DNA/RNA coding region at the nucleotide level. In the first graph, we plotted all the SE for all 498 DNA/RNA positions (Figure 3a). To know which nucleotide(s) was the driver of the DNA/RNA variability, we plotted the 166 first nucleotides of the env codon (called env N1; rev N2; tat/nef N3), the 166 second nucleotides of the env codon (called env N2; rev N3; tat/nef N1) and 166 third nucleotides of the env codon (env N3; rev N1; tat/nef N2;) in a separate graph (Figure 3b). We then plotted the SE of each polypeptide sequence at their equivalent expressed positions in a single graph (Figure 4). Finally, we analysed region by region the impact of this high concentration of overlapping ORF.
Figure 3. SE plots of the CT coding nucleotides.
We created a SE plot for all the nucleotides (A) or for each nucleotide by codon (B). In graph A and B, the line represents moving averages (30 positions for total sequence in black; 10 positions for the separated nucleotides): blue for the first nucleotides of each codon in the Env reading frame (env N1), red for the second nucleotides (env N2) and green for the third nucleotides (env N3). The N1 variability has a major impact on Env and Rev and a small impact on Tat/Nef, the N2 variability has a major impact on Env and Tat/Nef and a small impact on Rev and finally the env N3 variability has a major impact on Tat/Nef and Rev and a small impact on Env. Above each plot are shown key regions of the different proteins. A: The first conserved region containing the endocytosis signal. B: The region containing the Kennedy-like sequences. C: The hydrophobic region. D: LLP-2 region. E: LLP-3 region. F: LLP-3 / LLP-1 interegion. G: LLP-1 region + position 166 for the stop codon.
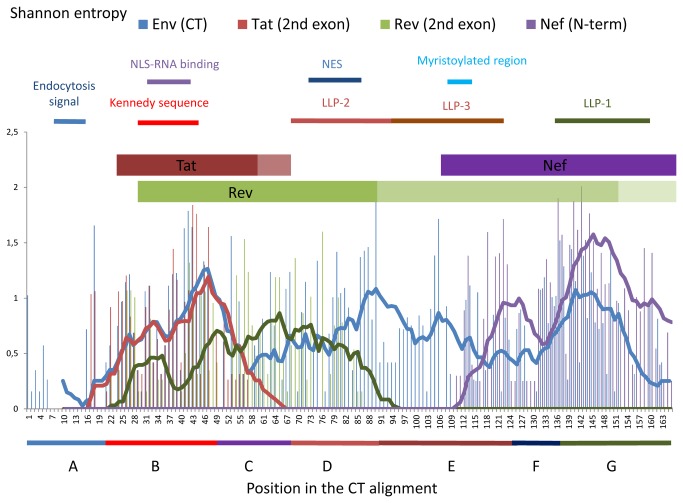
Figure 4. HIV-2 CT, Tat, Rev and Nef SE plot from our sequences.
Above each plot are shown key regions of the different proteins. The tat 2nd exon is expressed from the CT 16th -17th codon (16th CT position was chosen in the plot for the first Tat position; in brown) until the CT 51st - 52nd codon. Some tat 2nd exons continue to be expressed 6 codon later (shown in pale brown). The rev 2nd exon is expressed from the CT 20th -21st codon (20th CT position was chosen in the plot for the first Rev position) until the CT 104th - 105th codon (the last 14 Rev SE result are not shown due to the very complex situation created by insertion and deletion in this area; in green). The rev 2nd exon of the group B is expressed until the CT 151st -152nd codon (in pale green) and some group A rev 2nd exon continue to be expressed after the last CT codon (in faint green). nef is expressed from the CT 110th -111th codon (110th CT position was chosen in the plot for the first Nef position) and continue to be expressed after the last CT codon. The different coloured lines represent the moving average of 10 positions. NLS-RNA binding: nuclear localisation signal of Rev binding to the genomic RNA, NES: Rev nuclear export signal, myristoylated region: refers to Nef. Above the plot, key regions of the different proteins are shown. A: The first conserved region containing the endocytosis signal. B: The region containing the Kennedy-like sequences. C: The hydrophobic region. D: LLP-2 region. E: LLP-3 region. F: LLP-3 / LLP-1 interegion. G: LLP-1 region + position 166 for the stop codon.
In the coding sequence of the conserved first 20 CT aa positions, the DNA/RNA region contained mainly the env ORF (region A, Table 4) and had low variability. The small nucleotide variability of this region was due to the variability of the env N3 that have a low impact on the Env protein aa variability (corresponding predominantly to synonymous substitutions).
Table 4. Summary of the different SE analysed for each CT DNA/RNA coding regions.
| Region (CT coding region nucleotide position to nucleotide position) | Average SE of the entire nucleotides | Average SE env N1 | Average SE env N2 | Average SE env N3 |
|---|---|---|---|---|
| A (0 to 60) | 0.222 | 0.162 | 0.166 | 0.340 |
| B (61 to 141) | 0.416 | 0.261 | 0.624 | 0.359 |
| B group A* | 0.131 | 0.088 | 0.202 | 0.102 |
| B group B* | 0.086 | 0.050 | 0.135 | 0.073 |
| C (142 to 204) | 0.356 | 0.227 | 0.301 | 0.539 |
| C group A* | 0.084 | 0.055 | 0.071 | 0.128 |
| C group B* | 0.098 | 0.058 | 0.033 | 0.202 |
| D (205 to 273) | 0.476 | 0.320 | 0.637 | 0.470 |
| D/E (251 to 305) | 0.658 | 0.638 | 0.601 | 0.734 |
| E (274 to 375) | 0.492 | 0.482 | 0.380 | 0.613 |
| Nef (331 to 498) | 0.412 | 0.424 | 0.244 | 0.570 |
| Nef group A* | 0.114 | 0.116 | 0.058 | 0.169 |
| Nef group B* | 0.104 | 0.114 | 0.062 | 0.134 |
| F (376 to 418) | 0.407 | 0.519 | 0.190 | 0.365 |
| G (419 to 498) | 0.454 | 0.433 | 0.286 | 0.686 |
| G start (419 to 450) | 0.670 | 0.632 | 0.503 | 0.885 |
| G end (451 to 498) | 0.288 | 0.241 | 0.081 | 0.539 |
| CT (1 to 498) | 0.414 | 0.345 | 0.389 | 0.510 |
A: coding region of the 20 first conserved positions. B: coding region of the Kennedy-like region. C: coding region of the hydrophobic region. D: coding region of the LLP-2. E: coding region of the LLP-3. F: coding region of the LLP-3/LLP-1 inter-region. G: coding region of the LLP-1. D/E: complex region of the end of the LLP-2 and the beginning of the LLP-3. Nef: region where the Nef protein is expressed. * Group A and B SE were normalized with the natural log of the number of sequences.
Within the Kennedy-like region, we observed a contrast between the variability of the nucleotides and that of the aa. The variability of the aa in the CT region was the highest, while the variability of the nucleotides was relatively lower and was close to the average SE of the entire DNA CT coding region (region B, Table 4). This was due to the fact that only the env N2 drove the variability in this region. Because the env N1 and N3 (respectively rev N2 and N1) were conserved, the Rev protein was relatively constant (Figure 4). Finally, the only variability of the env N2 leads to the co-variation of the Env and Tat proteins (Figure 4).
The tat ORF ended five codons after the Kennedy-like coding region in most of our sequences, except for one sequence in which 6 codons were added to the consensus tat ORF (Genbank: KC748549). It is noteworthy that one tat ORF sequence was already finished at half the 2nd exon due to a premature stop codon (Genbank: KC748550). In the sequences coding for the most hydrophobic CT region and the LLP-2 regions (regions C and D, respectively, Table 4), the env N1 were mainly conserved compared to the average SE for regions C and D. By contrast, the env N3 were the most variable in the coding region for the conserved CT hydrophobic region, and the env N2 were the most variable in the CT LLP-2 coding region. Thus, in the env/rev overlapping regions we observed that: the env N1 were always conserved, the env N2 were variable when the env N3 were conserved and the env N3 were variable when the env N2 were conserved. In conclusion the Rev and Env aa positions varied independently and mostly in an opposite way. By homology with the reference strain ROD, the regions where Rev was conserved contained the Rev nuclear localization signal (NLS), the RNA binding domain (RBD) and the nuclear export signal (NES) [23].
After those regions, the rev ORF ended a few positions after the beginning of the LLP-3 coding sequence in most of our group A sequences, but continued in all the group B sequences. The group B Rev continued to be expressed up to the codons of the CT position 151/152. In addition, in two group A sequences we did not find any rev stop codon in the env sequence. The SE value was high at the beginning of the LLP-3 region coding sequences, mainly because of deletions and insertions (named D/E, Table 4). When Nef started to be expressed, at the end of the LLP-3 coding region, the variability was mainly driven by the first and third nucleotides of the env codons (named Nef, Table 4). Thus, the nucleotides that created the high Nef variability (env N3) had no consequence on Env variability, and the nucleotides that created variability in Env (env N1) had no consequence on Nef variability. At the end of the LLP-1, where Env CT was the most constant, the DNA/RNA variability of the corresponding coding sequene seemed to be driven only by the env N3, allowing Nef variability and Env conservation (G end, Table 4).
Differences between the HIV-2 groups A and B
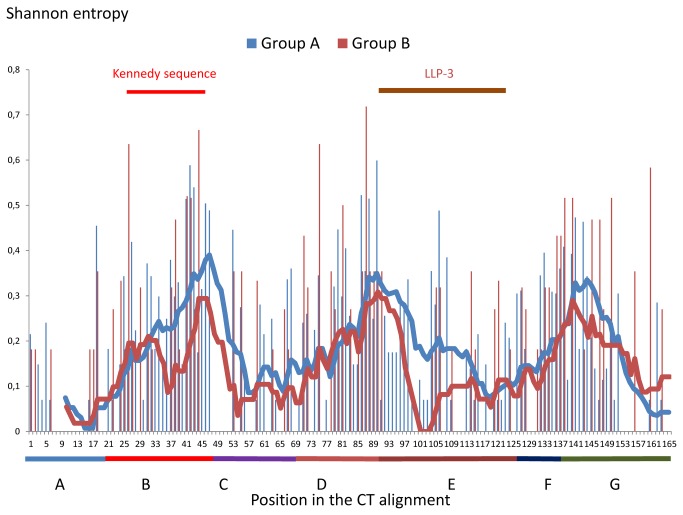
In the CT coding region, the SE (normalized by the natural log of the number of sequences) was similar between the HIV-2 groups A and B, except in the Kennedy-like region and at the beginning of the LLP-3 that was absent in sequences from group B. In the Kennedy-like region the mean SE was 50% higher in group A (Figure 5; region B group A and B Table 3), while the corresponding nucleotide sequence of the group A was more variable (region B group A and B, Table 4). This also means that the polypeptide expressed by the 2nd exon of tat was more conserved in group B. We also observed differences in the group B env N3, which were significantly more variable than the group A env N3 in the coding region of the hydrophobic region (region C group A and B, Table 4). However, this difference did not lead to differences in CT variability. Finally, in most of the group A sequences the 2nd exon of rev was two times smaller than its counterparts in group B creating an overlap with nef. This additional overlap had no consequence in the nucleotide variability (Nef group A and B, Table 4).
Figure 5. Normalised SE plot of the CT by HIV-2 group.
19 sequences from group A and 8 from group B are included. By contrast to the Figure 2, the SE are normalized by the natural log of the number of sequences to remove the entropy factor due to the sequences number. The lines represent the moving average of 10 positions (blue: group A, red: group B). The colours and letters below the plot represent the different studied regions: A: The first conserved region containing the endocytosis signal, B: The region containing the Kennedy-like sequences, C: The hydrophobic region, D: LLP-2 region, E: LLP-3 region, F: LLP-3 / LLP-1 inter-region, G: LLP-1 region + position 166 for the stop codon.
Discussion
We studied sequences of the HIV-2 Env C-terminal coding region from culture adapted strains and virus/provirus from clinical samples. We observed that stop codons were introduced prematurely in env in vitro but not in vivo. The position of the stop codon is interesting at two levels. First, it corresponds to a previously observed HIV-1 protease-cleavage site. In HIV-1, this site is important for resistance to the anti-fungus molecule Amphotericin B methyl ester (AME), which has an antiviral activity [24-26]. Furthermore, in the presence of AME, SIVmac selected the same stop codon than our HIV-2 strains [24]. But in the present study the selection of a premature stop codon happened without the presence of AME. Secondly the last aa of truncated Env is a proline, homologous to the first aa which anchors the HIV-1 Kennedy sequence in a model where the CT is partially outside the virus [1,14]. In our sequences, the region after this proline is very hydrophobic and mostly conserved. This suggests a potential membrane spanning region, and the presence of an external CT region similar to the HIV-1 Kennedy sequence. Some studies in HIV-1 showed the potential importance of the HIV-1 Env C-terminal part, corresponding to the HIV-2 Env C-terminal deleted part in cultured strains. This region was shown to play a role in the fusion process, the viral maturation, the packaging, and the anti-Env antibody recognition [27]. As the three first roles should act both in vitro and in vivo, the mandatory presence of the C-terminal region in vivo, suggests that immune recognition is the major selection pressure that favours a longer CT in vivo [40-42].
For practical reasons, it was not always possible to obtain a sequence either from viral RNA or proviral DNA in clinical samples. Global analysis could introduce a bias if both are very different. But our analysis detected the same variable and conserved regions in either RNA or DNA. The conserved regions are the endocytosis signal, the end of LLP-1 with the final di-leucine motif. The observed conservation of these regions is in line with their conservation among lentiviruses. The variable regions are the LLP-2, the Kennedy-like region and the beginning of the LLP-1. When compared to SIVmac, we found a supplementary conserved region known in SIVmac to be involved in NF-kB expression. However, that function is endorsed by another CT region in HIV-1, located in the LLP-2, which is not conserved between HIV types. It is not surprising to observe low HIV-1/HIV-2 conservation in the homologous LLP-2 and Kennedy sequences, as those two regions are already highly variable among HIV-1 groups and among our HIV-2 sequences [43]. Apart from being the least conserved regions, Kennedy-like region and LLP-2 region concentrated the mixtures of aa in population sequencing. This high variability can be explained because antibodies target those regions and they are therefore submitted to an evolutionary pressure [12–14,]. In contrast to the LLP-2, the Kennedy-like sequence is also variable in vitro when the sequences of cultured virus are compared with the reference sequences. This suggests that the Kennedy-like sequence is not exclusively variable in vivo while the LLP-2 is.
The HIV-2 DNA/RNA that encodes the variable Kennedy-like region encodes also the Rev conserved NLS and RBD. We showed that only the second nucleotides of env codons can vary to allow variability in Env with conservation in Rev (env N2= rev N3= tat N1). Consequently, the Kennedy-like region and the polypeptide expressed from the tat 2nd exon co-evolve while Rev is conserved. Interestingly, one patient had a tat premature stop codon in the DNA/RNA Kennedy-like coding region (polypeptide from the 2nd exon of tat shortened from 36 to 20 aa). This phenomenon does not seem to have an impact on virus replication since the patient had a high plasma viral load compared to the other patients (4th highest viral load (Genbank:KC748550); Table 2). Furthermore, a tat premature stop codon is also present in HIV-1 strain BRU without changing the viral replication capacity [45].
Several sequences of this series showed insertions or deletions at the beginning of the putative LLP-3 region. As seven different cases were observed in this set, the beginning of the LLP-3 seems to be a complex region. Furthermore, we observed that the same patient could harbour virus or provirus with different insertions/deletions. This implies that this insertion/deletion phenomenon is a dynamic process in patients. We did not find a clear link between the patient’s viral load and the presence of such insertion or deletion. However 3 of the 4 highest viral load values were linked to sequences with the longest insertion: (Genbank:KC748550), (Genbank:KC748555) and ((Genbank:KC748564); Table 2). Compared to the consensus, all sequences from group B shared a same deletion in that area while this was not observed in sequences from group A. The beginning of the CT LLP-3 complex coding region corresponded to the C-terminal region of Rev. Both proteins are therefore affected by the insertions and deletions found in our DNA/RNA sequences. We also observed that some of the rev ORF continued beyond the consensus stop. This was the case for our entire group B sequences and two group A sequences: (Genbank: KC748548) and (Genbank:KC748578). In the case of group B the size of the rev second exon was doubled. The high variability of that supplementary Rev C-terminal region does not plead in favour of an important role.
Finally, the end of the HIV-2 LLP-1 homologous region was highly conserved between our sequences and between primate lentiviruses. It was shown that this domain is implicated in Env incorporation within the envelope, HIV-1 infectivity, replication and fusion, and in neuronal cell death [37-39]. The high conservation between LLP-1 regions in primate lentiviruses implies that a similar effect could be found in SIV and HIV-2.
Conclusion
HIV-2 Env, Tat and Rev C-terminal regions are submitted to major length variations, either due to premature stop codons (only in vitro for Env and only in vivo for Tat), or to insertion and deletion inside the DNA/RNA sequences (for Env and Rev), or because of substitutions in the consensus stop codon (for Tat and Rev). The HIV-2 Env C-terminal part or CT contained three conserved and three variable regions. The conserved regions were the endocytosis signal and the regions homologous to the CT motif implied in SIVmac NF-kB expression and to the HIV-1 LLP-1 end. Two out of the 3 variable CT regions were homologous to HIV-1 Env regions harbouring epitope sequences: the Kennedy sequence and the lentiviral lytic peptide 2. Finally, this study highlighted that proteins expressed from the same nucleotide sequence in different reading frames mostly evolve independently as illustrated by Tat/Rev, Env/Rev and Env/Nef, but can also co-evolve as Tat and Env.
Materials and Methods
Ethical approval
The project was approved by the CEBHF (Comission d’Ethique Biomédicale Hospitalo-Facultaire) of the Université Catholique de Louvain, Brussels, Belgium (2010/18OCT/322, B-40320109572). A written informed consent was obtained from patient.
Strains and cell lines
The reference viruses ROD [46] and EHO [47] (obtained through the NIBSC, UK) were passaged several times on H9 cell lines (obtained through the NIH AIDS reagent reference program) [48-50]. The plasmid pKP59-ROD (used in [51]) was transfected on 293F cell lines (NIH AIDS reagent) [52]. The obtained virus was cultured several times on H9, MT2 and MT4 cell lines (NIH AIDS reagent) [53-57].
Extraction of the clinical samples
Blood samples were taken on EDTA from HIV-2 infected patients. Viral RNA was extracted from the plasma with the Nuclisens extraction kit (Biomérieux, Marcy l'Etoile, France). Proviral DNA was extracted from whole blood with the Nucleospin blood DNA extraction Kit (Macherey-Nagel; Düren, Germany).
Population Sequencing
We sequenced only the viral RNA for 17 of the patients, because the proviral DNA was not amplified for some or the samples were unavailable for the others. We sequenced both plasma RNA and provirus DNA in 4 of the patients. In 6 patients with low or undetectable viral load, only proviral DNA was sequenced. We developed a PCR amplification of the CT coding region with a nested PCR. Viral RNA or proviral DNA were amplified in a first reaction with the primers JR50 5’- CAGCAGGTTCTGCAATGGG -3’ and JR51 5’- CTCTCACTGTAATACATCCC -3’ at 300 nM, in a Master Mix with 2U of SuperScript-III enzyme (Life Technologies Ltd; Paisley, UK). The conditions used were 60 min at 45°C when the template was RNA, followed by 2 min at 94°C and 40 cycles of 30 sec at 94°C, 45 sec at 56°C, 2 min 30 à 72°C. A nested PCR using primers JR52 5’- TGGCCGGGATAGTGCAGC -3’ and JR53 5’- AACATCCCTTCCAGTCCC -3 at 300 nM was then performed using 2uL of the first PCR product with KOD enzyme master mix (Merck Millipore; Darmstadt, Germany). A touch-down protocol was used as follows: 2 min at 95°C and 10 cycles of 20 sec at 95°C, 10 sec 65°C to 56°C pendant (1°C less every cycle), 30 sec 70°C following by 30 cycles of 20 sec at 95°C, 10 sec 56°C, 30 sec 70°C.
The nested PCR products were purified with the QIAquick PCR purification kit (Qiagen; Venlo, Netherlands) and were sequenced with the primers JR49: 5’-GGTTTGACTTAACCTCCTGG-3’, JR52, JR53, JR54: 5’-GACAACAAGAACTGTTGCG-3’ and JR55: 5’-TGTCATTGGYCTYAGTGG-3’. We used the BigDye Terminator v3.1 (Applied Biosystems; Foster City, USA) for the sequencing reaction. The product was purified with the BigDye XTerminator Purification Kit (Applied Biosystems; Foster City, USA) and run in a capillary electrophoresis on the ABI3130 sequencing platform (Applied Biosystems; Foster City, USA).
Availability of supporting data
The data set supporting the results of this article are available in the Genbank repository. The accession numbers are from (Genbank:KC748535) to (Genbank:KC748580). For the “Analysis of the sequences”, “Influence of Tat, Rev and Nef on HIV-2 CT variability” and “Differences between the HIV-2 groups A and B” sections accession numbers of the sequences used can be found in Figure 1. For the “HIV-2 CT sequence variability over time in infected individuals” section accession numbers of sequences used were (by alignment): [(Genbank:KC748536) (Genbank:KC748537)], [(Genbank: KC748538) (Genbank:KC748539) (Genbank:KC748540) (Genbank:KC748541) (Genbank:KC748542) (Genbank:KC748543)], [(Genbank:KC748572) (Genbank:KC748573)], [(Genbank:KC748575) (Genbank:KC748576) (Genbank:KC748577)],[(Genbank:KC748545) (Genbank:KC748546)],[(Genbank:KC748567) (Genbank:KC748568)],[(Genbank:KC748553) (Genbank:KC748554)], [(Genbank:KC748569) (Genbank:KC748570) (Genbank:KC748571)] and [(Genbank:KC748560) (Genbank:KC748561)] For the “Provirus-virus sequences comparison” section accession numbers of the provirus sequences used in the alignment were (Genbank:KC748565), (Genbank:KC748566), (Genbank:KC748568), (Genbank:KC748569), (Genbank:KC748573), (Genbank:KC748574), (Genbank:KC748575), , (Genbank:KC748578), (Genbank:KC748579) and (Genbank:KC748580) and accession numbers of the virus sequences used In the alignment were (Genbank:KC748535), (Genbank:KC748536), (Genbank:KC748538), (Genbank:KC748544), (Genbank:KC748545), (Genbank:KC748547), (Genbank:KC748548), (Genbank:KC748549), (Genbank:KC748550), (Genbank:KC748551) (Genbank:KC748552), (Genbank:KC748553), (Genbank:KC748555), (Genbank:KC748556), (Genbank:KC748557), (Genbank:KC748558), (Genbank:KC748559), (Genbank:KC748560), (Genbank:KC748562), (Genbank:KC748563) and (Genbank:KC748564) (The numbers in italics represent the accession numbers of the sequences used for the direct provirus virus comparison).
Sequence analysis and alignment
Sequences were assembled and aligned using the IDNS database HIV-2 module (Smartgene; Zug, Switzerland). The program used for the multiple alignments was Multalin [58]. Shannon entropy was calculated by the program Entropy one [59]. The nucleotide sequences were translated by Transeq [60]. Finally, the Kyte Doolittle measure was performed in the Expasy portal [61].
Acknowledgments
We wish to thank particularly the different AIDS reference laboratories (ARL) in Belgium and CRP-Santé in Luxemburg who collected the samples, and the clinicians who cared for the patients. The patients are gratefully acknowledged for their acceptance to donate blood samples and to participate in the study. We thank all technicians and other personnel in the ARL of the Université catholique de Louvain who participated directly or indirectly in this study. We thank also Léonie GOEMINNE for her help in the sequencing and Philippe de Sany for his help with the in vitro study. The H9 cell line was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: H9 from Dr. Robert Gallo. The MT-2 cell line was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: MT-2 from Dr. Douglas Richman. The MT-4 cell line was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: MT-4 from Dr. Douglas Richman. The 293 cell line was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: 293 from Dr. Andrew Rice. HIV-2 ROD and EHO were obtained through the Programme EVA Centre for AIDS Reagents and came from the donors: Dr J-M Bechet ( ROD and EHO) and Professor L Montagnier (ROD). The pKP59-ROD was obtained from Dr Isabel Barahona from the Instituto Superior de Ciências da Saúde-Sul, Portugal. Finally, we are indebted to Sabine Hynes-Goubau for the final correction of this manuscript.
Funding Statement
NB is financed by the Fonds de la Recherche Scientifique (a public funding) url: http://www.frs-fnrs.be/. The funders (FNRS) had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Checkley MA, Luttge BG, Freed EO (2011) HIV-1 envelope glycoprotein biosynthesis, trafficking, and incorporation. J Mol Biol 410: 582-608. doi: 10.1016/j.jmb.2011.04.042. PubMed: 21762802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hallenberger S, Bosch V, Angliker H, Shaw E, Klenk HD et al. (1992) Inhibition of furin-mediated cleavage activation of HIV-1 glycoprotein gp160. Nature 360: 358–361. doi: 10.1038/360358a0. PubMed: 1360148. [DOI] [PubMed] [Google Scholar]
- 3. Bosch ML, Earl PL, Fargnoli K, Picciafuoco S, Giombini F et al. (1989) Identification of the fusion peptide of primate immunodeficiency viruses. Science 244: 694–697. doi: 10.1126/science.2541505. PubMed: 2541505. [DOI] [PubMed] [Google Scholar]
- 4. Weissenhorn W, Dessen A, Harrison SC, Skehel JJ, Wiley DC (1997) Atomic structure of the ectodomain from HIV-1 gp41. Nature 387: 426–430. doi: 10.1038/387426a0. PubMed: 9163431. [DOI] [PubMed] [Google Scholar]
- 5. Tan K, Liu J, Wang J, Shen S, Lu M (1997) Atomic structure of a thermostable subdomain of HIV-1 gp41. Proc Natl Acad, Sci U_S_A 94: 12303–12308. doi: 10.1073/pnas.94.23.12303. PubMed: 9356444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shang L, Yue L, Hunter E (2008) Role of the membrane-spanning domain of human immunodeficiency virus type 1 envelope glycoprotein in cell–cell fusion and virus infection. J Virol 82: 5417–5428. doi: 10.1128/JVI.02666-07. PubMed: 18353944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rowell JF, Stanhope PE, Siliciano RF (1995) Endocytosis of endogenously synthesized HIV-1 envelope protein, Mechanism and role in processing for association with class II MHC. J Immunol 155: 473–488. PubMed: 7602119. [PubMed] [Google Scholar]
- 8. Ohno H, Aguilar RC, Fournier MC, Hennecke S, Cosson P et al. (1997) Interaction of endocytic signals from the HIV-1 envelope glycoprotein complex with members of the adaptor medium chain family. Virology 238: 305–315. doi: 10.1006/viro.1997.8839. PubMed: 9400603. [DOI] [PubMed] [Google Scholar]
- 9. Boge M, Wyss S, Bonifacino JS, Thali M (1998) A membrane-proximal tyrosine-based signal mediates internalization of the HIV-1 envelope glycoprotein via interaction with the AP-2 clathrin adaptor. J Biol Chem 273: 15773–15778. doi: 10.1074/jbc.273.25.15773. PubMed: 9624176. [DOI] [PubMed] [Google Scholar]
- 10. Byland R, Vance PJ, Hoxie JA, Marsh M (2007) A conserved dileucine motif mediates clathrin and AP-2-dependent endocytosis of the HIV-1 envelope protein. Mol Cell Biol 18: 414–425. PubMed: 17108326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wyss S, Berlioz-Torrent C, Boge M, Blot G, Höning S et al. (2001) The highly conserved C-terminal dileucine motif in the cytosolic domain of the human immunodeficiency virus type 1 envelope glycoprotein is critical for its association with the AP-1 clathrin adaptor. J Virol 75: 2982–2992. doi: 10.1128/JVI.75.6.2982-2992.2001. PubMed: 11222723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kennedy RC, Henkel RD, Pauletti D, Allan JS, Lee TH et al. (1986) Antiserum to a synthetic peptide recognizes the HTLV-III envelope glycoprotein. Science 231: 1556–1559. doi: 10.1126/science.3006246. PubMed: 3006246. [DOI] [PubMed] [Google Scholar]
- 13. Chanh TC, Dreesman GR, Kanda P, Linette GP, Sparrow JT et al. (1986) Induction of anti-HIV neutralizing antibodies by synthetic peptides. EMBO J 5: 3065–3071. PubMed: 3466790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cleveland SM, McLain L, Cheung L, Jones TD, Hollier M et al. (2003) A region of the C-terminal tail of the gp41 envelope glycoprotein of human immunodeficiency virus type 1 contains a neutralizing epitope: evidence for its exposure on the surface of the virion. J Gen Virol 84: 591–602. doi: 10.1099/vir.0.18630-0. PubMed: 12604810. [DOI] [PubMed] [Google Scholar]
- 15. Miller MA, Garry RF, Jaynes JM, Montelaro RC (1991) A structural correlation between lentivirus transmembrane proteins and natural cytolytic peptides. AIDS Res Hum Retrovir 7: 511–519. doi: 10.1089/aid.1991.7.511. PubMed: 1657072. [DOI] [PubMed] [Google Scholar]
- 16. Miller MA, Cloyd MW, Liebmann J, Rinaldo CR, Islam KR et al. (1993) Alterations in cell membrane permeability by the lentivirus lytic peptide (LLP-1) of HIV-1 transmembrane protein. Virology 196: 89–100. doi: 10.1006/viro.1993.1457. PubMed: 8356808. [DOI] [PubMed] [Google Scholar]
- 17. Eisenberg D, Wesson M (1990) The most highly amphiphilic alpha-helices include two aa segments in human immunodeficiency virus glycoprotein 41. Biopolymers 29: 171–177. doi: 10.1002/bip.360290122. PubMed: 2328285. [DOI] [PubMed] [Google Scholar]
- 18. Venable RM, Pastor RW, Brooks BR, Carson FW (1989) Theoretically determined three-dimensional structures for amphipathic segments of the HIV-1 gp41 envelope protein. AIDS Res Hum Retrovir 5: 7–22. doi: 10.1089/aid.1989.5.7. PubMed: 2541749. [DOI] [PubMed] [Google Scholar]
- 19. Noble B, Abada P, Nunez-Iglesias J, Cannon PM (2006) Recruitment of the adaptor protein 2 complex by the human immunodeficiency virus type 2 envelope protein is necessary for high levels of virus release. J Virol 80: 2924-2932. doi: 10.1128/JVI.80.6.2924-2932.2006. PubMed: 16501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Naidu YM, Kestler HW III, Li Y, Butler CV, Silva DP et al. (1988) Characterization of infectious molecular clones of simian immunodeficiency virus (SIVmac) and human immunodeficiency virus type 2: persistent infection of rhesus monkeys with molecularly cloned SIVmac . J Virol 62: 4691-4696. PubMed: 2846880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schmitt AO, Herzel H (1997) Estimating the entropy of DNA sequences. J Theor Biol, 188: 369-377. doi: 10.1006/jtbi.1997.0493. PubMed: 9344742. [DOI] [PubMed] [Google Scholar]
- 22. Postler TS, Desrosiers RC (2012) The cytoplasmic domain of the HIV-1 glycoprotein gp41 induces NF-κB activation through TGF-β-activated kinase 1. Cell Host Microbe 11: 181-193. doi: 10.1016/j.chom.2011.12.005. PubMed: 22341466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dillon PJ, Nelbock P, Perkins A, Rosen CA (1990) Function of the human immunodeficiency virus types 1 and 2 Rev proteins is dependent on their ability to interact with a structured region present in env gene mRNA. J Virol 64: 4428-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Waheed AA, Ablan SD, Mankowski MK, Cummins JE, Ptak RG et al. (2006) Inhibition of HIV-1 replication by amphotericin B methyl ester: selection for resistant variants. J Biol Chem 281: 28699–28711. doi: 10.1074/jbc.M603609200. PubMed: 16882663. [DOI] [PubMed] [Google Scholar]
- 25. Waheed AA, Ablan SD, Roser JD, Sowder RC, Schaffner CP et al. (2007) HIV-1 escape from the entry-inhibiting effects of a cholesterol-binding compound via cleavage of gp41 by the viral protease. Proc Natl Acad Sci U_S_A 104: 8467–8471. doi: 10.1073/pnas.0701443104. PubMed: 17483482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Waheed AA, Ablan SD, Sowder RC, Roser JD, Schaffner CP et al. (2010) Effect of mutations in the human immunodeficiency virus type 1 protease on cleavage of the gp41 cytoplasmic tail. J Virol 84: 3121–3126. doi: 10.1128/JVI.02002-09. PubMed: 20042499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee SJ, Hu W, Fisher AG, Looney DJ, Kao VF et al. (1989) Role of the carboxy-terminal portion of the HIV-1 transmembrane protein in viral transmission and cytopathogenicity. AIDS Res Hum Retrovir 5: 441–449. doi: 10.1089/aid.1989.5.441. PubMed: 2788444. [DOI] [PubMed] [Google Scholar]
- 28. Wilk T, Pfeiffer T, Bosch V (1992) Retained in vitro infectivity and cytopathogenicity of HIV-1 despite truncation of the C-terminal tail of the env gene product. Virology 189: 167–177. doi: 10.1016/0042-6822(92)90692-I. PubMed: 1604808. [DOI] [PubMed] [Google Scholar]
- 29. Gabuzda DH, Lever A, Terwilliger E, Sodroski J (1992) Effects of deletions in the cytoplasmic domain on biological functions of human immunodeficiency virus type 1 envelope glycoproteins. J Virol 66: 3306–3315. PubMed: 1583717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yu X, Yuan X, McLane MF, Lee TH, Essex M (1993) Mutations in the cytoplasmic domain of human immunodeficiency virus type 1 transmembrane protein impair the incorporation of Env proteins into mature virions. J Virol 67: 213–221. PubMed: 8416370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Freed EO, Martin MA (1995) Virion incorporation of envelope glycoproteins with long but not short cytoplasmic tails is blocked by specific, single aa substitutions in the human immunodeficiency virus type 1 matrix. J Virol 69: 1984–1989. PubMed: 7853546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Freed EO, Martin MA (1996) Domains of the human immunodeficiency virus type 1 matrix and gp41 cytoplasmic tail required for envelope incorporation into virions. J Virol 70: 341–351. PubMed: 8523546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Murakami T, Freed EO (2000) Genetic evidence for an interaction between human immunodeficiency virus type 1 matrix and alpha-helix 2 of the gp41 cytoplasmic tail. J Virol 74: 3548–3554. doi: 10.1128/JVI.74.8.3548-3554.2000. PubMed: 10729129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kalia V, Sarkar S, Gupta P, Montelaro RC (2003) Rational site-directed mutations of the LLP-1 and LLP-2 lentivirus lytic peptide domains in the intracytoplasmic tail of human immunodeficiency virus type 1 gp41 indicate common functions in cell-cell fusion but distinct roles in virion envelope incorporation. J Virol 77: 3634–3646. doi: 10.1128/JVI.77.6.3634-3646.2003. PubMed: 12610139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Affranchino JL, González SA (2006) Mutations at the C-terminus of the simian immunodeficiency virus envelope glycoprotein affect gp120-gp41 stability on virions. Virology 347: 217–225. doi: 10.1016/j.virol.2005.11.032. PubMed: 16380144. [DOI] [PubMed] [Google Scholar]
- 36. Kalia V, Sarkar S, Gupta P, Montelaro RC (2003) Rational site-directed mutations of the LLP-1 and LLP-2 lentivirus lytic peptide domains in the intracytoplasmic tail of human immunodeficiency virus type 1 gp41 indicate common functions in cell-cell fusion but distinct roles in virion envelope incorporation. J Virol 77: 3634-3646. doi: 10.1128/JVI.77.6.3634-3646.2003. PubMed: 12610139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Newman JT, Sturgeon TJ, Gupta P, Montelaro RC (2007) Differential functional phenotypes of two primary HIV-1 strains resulting from homologous point mutations in the LLP domains of the envelope gp41 intracytoplasmic domain. Virology 367: 102-116. doi: 10.1016/j.virol.2007.05.027. PubMed: 17582453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sung JH, Shin SA, Park HK, Montelaro RC, Chong YH (2001) Protective effect of glutathione in HIV-1 lytic peptide 1-induced cell death in human neuronal cells. J Neurovirol 7: 454-465. doi: 10.1080/135502801753170318. PubMed: 11582518. [DOI] [PubMed] [Google Scholar]
- 39. Yang P, Ai LS, Huang SC, Li HF, Chan WE et al. (2010) The cytoplasmic domain of human immunodeficiency virus type 1 transmembrane protein gp41 harbors lipid raft association determinants. J Virol 84: 59-75. doi: 10.1128/JVI.00899-09. PubMed: 19793805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Spies CP, Ritter GD Jr, Mulligan MJ, Compans RW (1994) Truncation of the cytoplasmic domain of the simian immunodeficiency virus envelope glycoprotein alters the conformation of the external domain. J Virol 68: 585-591. PubMed: 8289362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Edwards TG, Wyss S, Reeves JD, Zolla-Pazner S, Hoxie JA et al. (2002) Truncation of the cytoplasmic domain induces exposure of conserved regions in the ectodomain of human immunodeficiency virus type 1 envelope protein. J Virol 76: 2683-2691. doi: 10.1128/JVI.76.6.2683-2691.2002. PubMed: 11861835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kalia V, Sarkar S, Gupta P, Montelaro RC (2005) Antibody neutralization escape mediated by point mutations in the intracytoplasmic tail of human immunodeficiency virus type 1 gp41. J Virol 79: 2097-2107. doi: 10.1128/JVI.79.4.2097-2107.2005. PubMed: 15681412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Steckbeck JD, Craigo JK, Barnes CO, Montelaro RC (2011) Highly conserved structural properties of the C-terminal tail of HIV-1 gp41 protein despite substantial sequence variation among diverse clades: implications for functions in viral replication. J Biol Chem 286: 27156-27166. doi: 10.1074/jbc.M111.258855. PubMed: 21659530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lu L, Zhu Y, Huang J, Chen X, Yang H et al. (2008) Surface exposure of the HIV-1 env cytoplasmic tail LLP2 domain during the membrane fusion process: interaction with gp41 fusion core. J Biol Chem 283: 16723-16731. doi: 10.1074/jbc.M801083200. PubMed: 18408000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jeang KT, Xiao H, Rich EA (1999) Multifaceted activities of the HIV-1 transactivator of transcription, Tat. J Biol Chem, 274: 28837-28840. doi: 10.1074/jbc.274.41.28837. PubMed: 10506122. [DOI] [PubMed] [Google Scholar]
- 46. Clavel F, Guétard D, Brun-Vézinet F, Chamaret S, Rey MA et al. (1986) Isolation of a new human retrovirus from West African patients with AIDS. Science, 233: 343-346. doi: 10.1126/science.2425430. PubMed: 2425430. [DOI] [PubMed] [Google Scholar]
- 47. Rey MA, Krust B, Laurent AG, Guétard D, Montagnier L et al. (1989) Characterization of an HIV-2-related virus with a smaller sized extracellular envelope glycoprotein. Virology, 173: 258-267. doi: 10.1016/0042-6822(89)90242-0. PubMed: 2683362. [DOI] [PubMed] [Google Scholar]
- 48. Mann DL, O'Brien SJ, Gilbert DA, Reid Y, Popovic M et al. (1989) Origin of the HIV-susceptible human CD4+ cell line H9. AIDS Res Hum Retrovir 5: 253-255. doi: 10.1089/aid.1989.5.253. PubMed: 2567177. [DOI] [PubMed] [Google Scholar]
- 49. Popovic M, Read-Connole E, Gallo RC (1984) T4 positive human neoplastic cell lines susceptible to and permissive for HTLV-III. Lancet 2: 1472-1473. PubMed: 6151082. [DOI] [PubMed] [Google Scholar]
- 50. Popovic M, Sarngadharan MG, Read E, Gallo RC (1984) Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science, 224: 497-500. doi: 10.1126/science.6200935. PubMed: 6200935. [DOI] [PubMed] [Google Scholar]
- 51. Ribeiro AC, Maia e Silva A, Santa-Marta M, Pombo A, Moniz-Pereira J et al. (2005) Functional analysis of Vif protein shows less restriction of human immunodeficiency virus type 2 by APOBEC3G. J Virol, 79: 823-833. doi: 10.1128/JVI.79.2.823-833.2005. PubMed: 15613310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Graham FL, Smiley J, Russell WC, Nairn R (1977) Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol 36: 59-74. doi: 10.1099/0022-1317-36-1-59. PubMed: 886304. [DOI] [PubMed] [Google Scholar]
- 53. Haertle T, Carrera CJ, Wasson DB, Sowers LC, Richman DD et al. (1988) Metabolism and anti-human immunodeficiency virus-1 activity of 2-halo-2', 3'-dideoxyadenosine derivatives. J Biol Chem 263: 5870-5875. PubMed: 3258602. [PubMed] [Google Scholar]
- 54. Harada S, Koyanagi Y, Yamamoto N (1985) Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science 229: 563-566. doi: 10.1126/science.2992081. PubMed: 2992081. [DOI] [PubMed] [Google Scholar]
- 55. Harada S, Koyanagi Y, Yamamoto N (1985) Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science 229: 563-566. doi: 10.1126/science.2992081. PubMed: 2992081. [DOI] [PubMed] [Google Scholar]
- 56. Larder BA, Darby G, Richman DD (1989) HIV with reduced sensitivity to Zidovudine (AZT) isolated during prolonged therapy. Science 243: 1731-1734. doi: 10.1126/science.2467383. PubMed: 2467383. [DOI] [PubMed] [Google Scholar]
- 57. Pauwels R, De Clercq E, Desmyter J, Balzarini J, Goubau P et al. (1987) Sensitive and rapid assay on MT-4 cells for detection of antiviral compounds against the AIDS virus. J Virol Methods 16: 171-185. doi: 10.1016/0166-0934(87)90002-4. PubMed: 2821048. [DOI] [PubMed] [Google Scholar]
- 58. Multalin. Available: http://npsa-pbil.ibcp.fr/cgi-bin/npsa_automat.pl?page=npsa_multalin.html. Accessed 29 May 2013.
- 59.http://www.hiv.lanl.gov/content/sequence/ENTROPY/entropy_one.html Entropy one. Available: . Accessed: 29 May 2013.
- 60. Transeq. Available: http://www.ebi.ac.uk/Tools/st/emboss_transeq/. Accessed 29 May 2013.
- 61. Kyte & Doolittle. Available: http://web.expasy.org/protscale/. Accessed 29 May 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data set supporting the results of this article are available in the Genbank repository. The accession numbers are from (Genbank:KC748535) to (Genbank:KC748580). For the “Analysis of the sequences”, “Influence of Tat, Rev and Nef on HIV-2 CT variability” and “Differences between the HIV-2 groups A and B” sections accession numbers of the sequences used can be found in Figure 1. For the “HIV-2 CT sequence variability over time in infected individuals” section accession numbers of sequences used were (by alignment): [(Genbank:KC748536) (Genbank:KC748537)], [(Genbank: KC748538) (Genbank:KC748539) (Genbank:KC748540) (Genbank:KC748541) (Genbank:KC748542) (Genbank:KC748543)], [(Genbank:KC748572) (Genbank:KC748573)], [(Genbank:KC748575) (Genbank:KC748576) (Genbank:KC748577)],[(Genbank:KC748545) (Genbank:KC748546)],[(Genbank:KC748567) (Genbank:KC748568)],[(Genbank:KC748553) (Genbank:KC748554)], [(Genbank:KC748569) (Genbank:KC748570) (Genbank:KC748571)] and [(Genbank:KC748560) (Genbank:KC748561)] For the “Provirus-virus sequences comparison” section accession numbers of the provirus sequences used in the alignment were (Genbank:KC748565), (Genbank:KC748566), (Genbank:KC748568), (Genbank:KC748569), (Genbank:KC748573), (Genbank:KC748574), (Genbank:KC748575), , (Genbank:KC748578), (Genbank:KC748579) and (Genbank:KC748580) and accession numbers of the virus sequences used In the alignment were (Genbank:KC748535), (Genbank:KC748536), (Genbank:KC748538), (Genbank:KC748544), (Genbank:KC748545), (Genbank:KC748547), (Genbank:KC748548), (Genbank:KC748549), (Genbank:KC748550), (Genbank:KC748551) (Genbank:KC748552), (Genbank:KC748553), (Genbank:KC748555), (Genbank:KC748556), (Genbank:KC748557), (Genbank:KC748558), (Genbank:KC748559), (Genbank:KC748560), (Genbank:KC748562), (Genbank:KC748563) and (Genbank:KC748564) (The numbers in italics represent the accession numbers of the sequences used for the direct provirus virus comparison).