Abstract
Dihydroxyphenylalanine (DOPA) is a neutral amino acid that resembles natural l-dopa (dopamine precursor). It enters the catecholamine metabolic pathway of endogenous l-DOPA in the brain and peripheral tissues. It is amenable to labeling with fluorine-18 (18F) for PET imaging and was originally used in patients with Parkinson’s disease to assess the integrity of the striatal dopaminergic system. The recent introduction and use of hybrid PET/CT scanners has contributed significantly to the management of a series of other pathologies including neuroendocrine tumors, brain tumors, and pancreatic cell hyperplasia. These pathologic entities present an increased activity of l-DOPA decarboxylase and therefore demonstrate high uptake of 18F-DOPA. Despite these potentially promising applications in several clinical fields, the role of 18F-DOPA has not been elucidated completely yet because of associated difficulties in synthesis and availability. Unfortunately, the available literature does not provide recommendations for procedures or administered activity, acquisition timing, and premedication with carbidopa. The aim of this paper is to outline the physiological biodistribution and normal variants, including possible pitfalls that may lead to misinterpretations of the scans in various clinical settings.
Keywords: biodistribution; 18F-DOPA; 18F-DOPA pitfalls; 18F-DOPA variants; l-6-fluoro-3,4-dihydroxyphenylalanine PET/CT; physiologic pattern
Introduction
The introduction of PET imaging with 18F-fluorodeoxyglucose (18F-FDG) and continuous developments in hybrid PET/computed tomography (CT) systems have considerably improved the management of a wide range of oncologic and nononcologic pathologies. However, the search for new and more specific PET radiopharmaceuticals continues because of the documented limitations of 18F-FDG. These include its inability to detect well-differentiated tumors such as neuroendocrine tumors (NETs), to differentiate inflammation from neoplasms, to reveal lesions in organs with high physiological uptake, such as brain tumors, or to assess more specific metabolic processes other than glycolytic metabolism 1. One of these new and promising radiotracers is the amino acid-based radiopharmaceutical l-6-[18F]fluoro-3,4-dihydroxyphenylalanine (18F-DOPA), which was originally used in patients with Parkinson’s disease to assess the integrity of the striatal dopaminergic system. In recent years, especially after the introduction of hybrid PET/CT scanners, 18F-DOPA has acquired an important role in the management of NETs and brain tumors and in the evaluation of pancreatic cell hyperplasia 2–5.
However, despite the well-defined pathophysiological basis and the promising diagnostic results of 18F-DOPA PET, there still remains a relative paucity of data because of the rarity of these pathologies, the continued use of other PET/SPET radiopharmaceuticals, and, more importantly, the complicated production of 18F-DOPA 6,7.
The purpose of this paper is to highlight the main clinical applications of 18F-DOPA PET/CT and, focusing on the physiological biodistribution of the tracer and its normal variants, describe possible pitfalls that may lead to misinterpretations of the scans in the various clinical settings.
The radiopharmaceutical
DOPA is an amino acid containing two hydroxyl groups on the third and fourth positions of the phenol ring, and it can be labeled with the positron emitter isotope 18F in the sixth position, forming 18F-DOPA, which allows PET imaging 8.
l-DOPA is the precursor of the neurotransmitters dopamine, epinephrine (adrenaline), and norepinephrine (noradrenaline), collectively known as catecholamines. 18F-DOPA is a large neutral amino acid that resembles natural l-DOPA biochemically and has similar kinetics. It enters the catecholamine metabolic pathway of endogenous l-DOPA both in the brain and peripherally 9,10. The relatively long half-life (110 min) of 18F-DOPA makes it suitable for transportation to centers with no cyclotron facility on site, allowing for more ‘flexible’ imaging timings, and offers the possibility of late images, which are very important in some cases.
The main clinical application of imaging with 18F-DOPA PET/CT is for the assessment of the striatum, brain tumors, NETs, and congenital hyperinsulinemic hypoglycemia.
18F-DOPA PET/CT acquisition protocol
Preparation
Most centers that perform 18F-DOPA PET/CT ask patients to fast for 4–6 h before they are administered the intravenous injection of 18F-DOPA. Medication that could interfere with the uptake of the tracer can be withdrawn for 24 h; however, for the majority of indications no special interactions have been reported and no suspension of pharmaceuticals is needed. Some centers premedicate patients with carbidopa (usually 100–200 mg 1 h before the injection). The only specific preparation described in the literature is for congenital hyperinsulinism in which investigators from several centers have proposed a standard protocol for performing 18F-DOPA PET/CT, which consists of discontinuing some medications (diazoxide, octreotide, and glucagon) for 2 days, fasting for 6 h, and a glucose infusion to maintain euglycemia. In this case, the use of carbidopa is not recommended because it could block not only the physiological pancreatic uptake but also uptake in the pathological area within the pancreas 11.
Dose and timing
The activity administered varies largely in the literature. In our center we administer a fixed dose of 185 MBq of 18F-DOPA, and all acquisitions are performed 1 h after injection. The choice of a 1 h time interval between injection and acquisition was made on the basis of a previous preclinical study that we had performed on 15 Macacus monkeys as part of the Xenome multicenter European Community Project. During that study, we observed that the steady state of the pharmaceutical in the striatum was reached at 1 h after injection 12. The tracer should be injected slowly, to avoid pharmacologic effects, which could provoke a carcinoid crisis, especially when specific activity is low 13. Other suggested doses and acquisition timings have been described: in patients with NETs, Kauhanen et al. 2 reported an average administered dose of 234±56 MBq and acquisition at 1 h after injection. Schiesser et al. 14 used standard doses of 200–220 MBq and performed acquisition at 45 min. For recurrent medullary thyroid carcinoma (MTC), variable doses (185–740 MBq), acquisition timing (50–90 min), and time per bed position (3–20 min) have been reported 14 with and without carbidopa premedication 15. Similar variations were also shown in the studies included in a recent meta-analysis of paraganglioma (PGL) with mean doses of 180–470 MBq, acquisition timing of 30–90 min, and carbidopa premedication 16. The EANM guidelines on PGL imaging recommend a dose of 4 MBq/kg and imaging 30–60 min after injection 17. In the evaluation of brain tumors the best time interval for acquisition is between 10 and 30 min after injection, as tumor uptake is near maximum and occurs sufficiently early to avoid peak uptake in the striatum 3.
Acquisition
Imaging with 18F-DOPA is similar to that performed with most PET radiopharmaceuticals, extending from the base of the skull to mid-thigh, or over the whole body, depending on the clinical requirement. Early images centered over the abdomen may be acquired to overcome difficulties in localizing abdominal PGL located near the hepatobiliary system because of physiological tracer elimination 17. Early images centered over the neck may also be acquired in patients with MTC, as these tumors often show rapid washout and are better visualized on these early images 18,19.
The use of hybrid PET/CT scanners has become widespread in recent years, with the whole-body protocol consisting of a scout view, followed by low-dose CT acquisition for attenuation correction and subsequent PET acquisition, with parameters depending on the scanner used and hospital protocols. Whole-body acquisition usually lasts for 15–30 min (5–7 bed positions of 3–5 min each).
The relatively long half-life of the tracer (110 min) also allows for late specific spot images that can be very useful in case of interpretative doubts (e.g. activity in the urinary system or in the bowel).
In our center, in the case of patients who are referred for MTC or those with or suspected of having head and neck disease, an additional late acquisition of the head and neck region is routinely performed using a specific acquisition protocol from the vertex to the upper lung, with the same whole-body scout and CT parameters but with different PET parameters (two bed positions of 5 min each and a matrix of 256×256). The bigger matrix and the longer PET acquisition protocol offer a better spatial resolution and a better identification of small pathological deposits with faint uptake (e.g. small laterocervical lymph nodes). This will also improve the quality of images in patients with frequent head and neck movement. Assessment of specific areas such as the lower lung, upper abdomen, and the pelvis may require additional ‘motion free’ or respiratory gating acquisition to aid in image interpretation.
Normal biodistribution of whole-body 18F-DOPA PET/CT
Knowledge of the physiological uptake of radiopharmaceuticals, as well of their distribution and normal variants, represents an important step toward the correct interpretation of pathological findings. In 2011, our group published a study on the physiological biodistribution pattern and the physiological variants of 18F-DOPA PET/CT in a cohort comprising 107 patients (53 men and 54 women; mean age 54.6 years, range 9–85 years) 20. Patients were referred to our center for suspected NETs, mainly pheochromocytoma (PCC), PGL, and MTC. PET scans were acquired without premedication with carbidopa. A semiquantitative uptake analysis using the upper limit of the standardized uptake value (SUVmax) at the sites of physiological uptake was performed in both 18F-DOPA-negative (n=32) and 18F-DOPA-positive (n=75) patients using a planar circular region of interest of 1 cm diameter, automatically generated by the computer, positioned on the slice with the highest uptake of each organ.
All patients demonstrated uptake in the basal ganglia (mean SUVmax 2.77, range 1.5–3.6), liver (mean SUVmax 2.15, range 1.1–2.9), pancreas [mainly in the uncinate process (mean SUVmax 5.67, range 2.9–14.1) and less intense in the body–tail region (mean SUVmax 4.07, range 2.1–6.2)], and adrenal glands (mean SUVmax 1.92, range 0.7–4.3). Very intense and variable uptake was seen in the excretory organs – gallbladder and biliary tract (mean SUVmax 8.5), kidneys (mean SUVmax 4.4), and ureters and urinary bladder (mean SUVmax 111) – depending on individual elimination timing. In our series, bowel uptake (mean SUVmax 2.3) was an unusual finding and when seen presented only mild diffuse uptake. Statistical analysis using the Mann–Whitney test showed that the physiological biodistribution of DOPA was not significantly different between the DOPA-positive and DOPA-negative scans (P>0.05). Mild uptake could also be seen in the liver, myocardium, and peripheral muscles; in some cases, very faint uptake could be seen in the mammary glands, the oral cavity, the esophagus, the duodenum, and the bowel. The uptake was mildly variable in the basal ganglia and even less variable in the liver parenchyma. However, greater variability was observed in the pancreas, especially in the uncinate process, and in the adrenal glands. Regarding the adrenals, our data showed significant variability in DOPA uptake. Physiological DOPA uptake was observed in 44% of normal patients with a relatively high SUVmax of up to 4.3. However, morphological imaging and metanephrine urinary levels were normal in all these cases, except in the case of medullary adrenal disease. However, care should be taken while interpreting uptake in these glands. It is reported in the literature that uptake in the growth plates is common in the pediatric population 8. In Figs 1–3 are shown some examples of the physiological variability of 18F-DOPA uptake in various organs and in some pathological lesions.
Fig. 1.
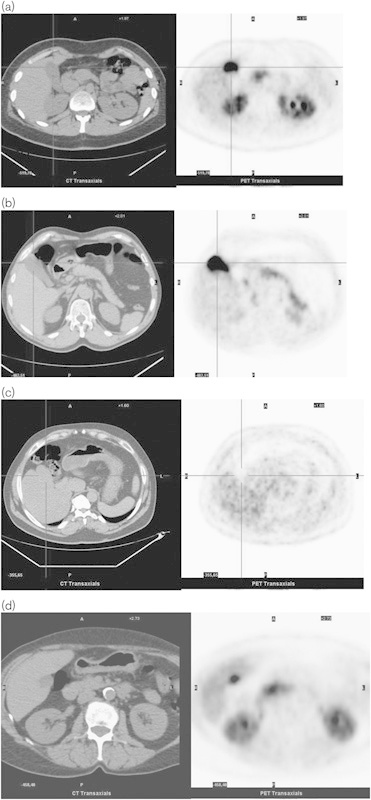
Normal variants of l-6-[18F]fluoro-3,4-dihydroxyphenylalanine (18F-DOPA) uptake in the gallbladder. (a–d) On the left: low-dose computed tomography (CT) transaxial images; on the right: PET images. Images without carbidopa premedication. (a) Intense uptake in the gallbladder. The 18F-DOPA uptake is high in the kidneys as well. Note the focal uptake on the head of the pancreas. (b) Intense uptake in the gallbladder. Note the diffuse 18F-DOPA uptake of the body–tail of the pancreas and the mild uptake of the adrenals, especially the right one. (c) Absent uptake in the gallbladder. (d) Focal intense uptake in the gallbladder that, because of the position, could be misinterpreted as a hepatic lesion. Uptake is high in the kidneys as well. Note the uptake in the head of the pancreas.
Fig. 2.
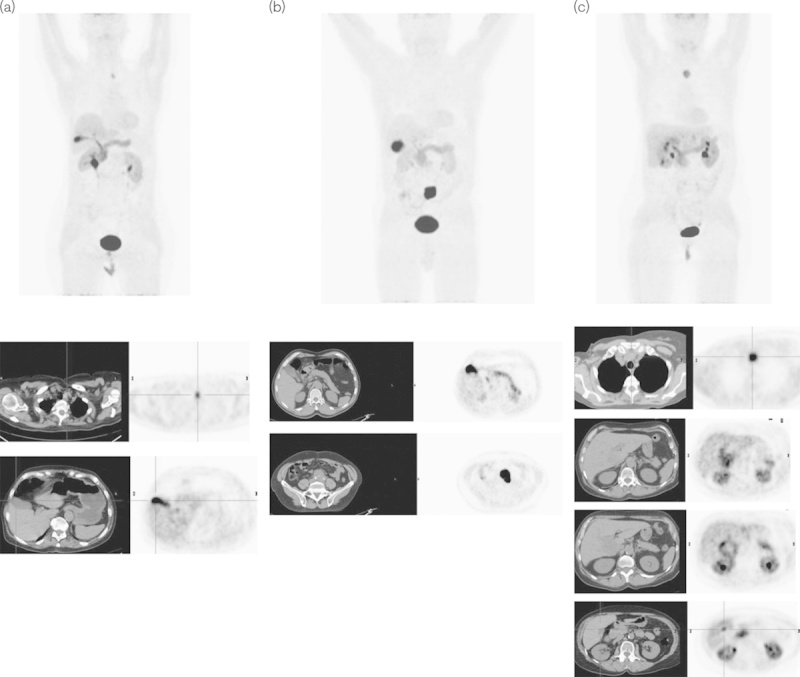
Normal variants of the l-6-[18F]fluoro-3,4-dihydroxyphenylalanine (18F-DOPA) excretion through the biliary tract. Upper series: MIP images of three patients (a–c) with medullary thyroid carcinoma (MTC) (a, c) and paraganglioma (b). Lower series: axial images of the low-dose computed tomography (CT) (on the left) and PET (on the right) of the pathologic uptake of 18F-DOPA. (a) a 78-year-old woman with MTC previously operated upon and with suspicion of relapse in the left paratracheal region well visualized at 18F-DOPA PET/CT; (b) a 68-year-old man with a suspicion of paraganglioma on morphological imaging in the aortic carefour region of the abdomen confirmed on 18F-DOPA PET/CT, which depicted an area of very high radiotracer uptake with an SUVmax of 29.9; (c) a 66-year-old woman with MTC previously operated upon, with increased calcitonin levels and hepatic and nodal metastases. In all of the above images, the variability of 18F-DOPA uptake in the physiological sites and pathologic areas, which are clearly recognized, is noteworthy. Note the very intense uptake in the gallbladder in (a) and (b), and the uptake in the biliary tracts especially in (a) and (c). In (c) note the lesions in the liver as well as the physiologic focal uptake in the gallbladder that could be misinterpreted as another hepatic lesion.
Fig. 3.
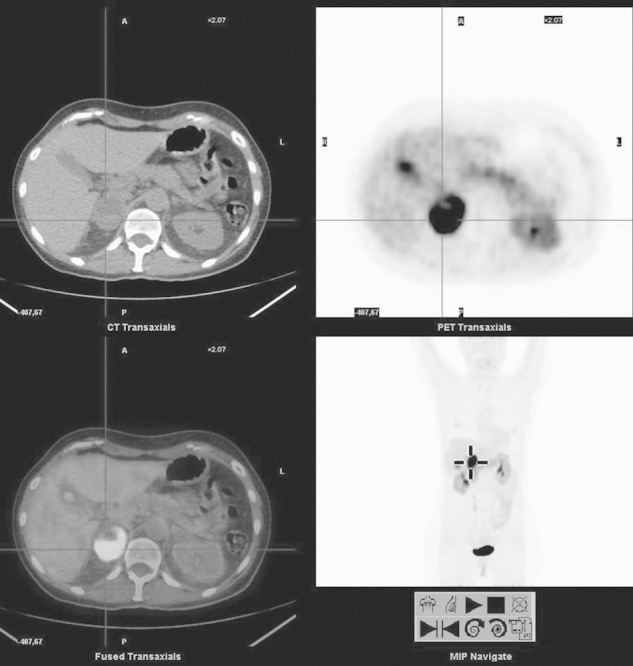
Right adrenal lesion. From the upper left, clockwise: computed tomography (CT), PET, MIP, and fused PET/CT images of l-6-[18F]fluoro-3,4-dihydroxyphenylalanine (18F-DOPA) PET/CT of a 51-year-old woman presenting with very high urinary normetanephrin levels. 123I-MIBG scintigraphy revealed only a mild to moderate uptake of the tracer in the right adrenal. Instead, 18F-DOPA PET/CT shows a very intense uptake in the right adrenal, consistent with pheochromocytoma. Note the concomitant uptake in the body–tail of the pancreas, and the intense uptake in the gallbladder. Images with no carbidopa premedication.
Pretreatment with carbidopa
Carbidopa [l-α-hydrazino-α-methyl-β-(3,4-dihydroxyphenyl) propionic acid] is a known inhibitor of aromatic amino acid decarboxylase, and it inhibits the conversion of l-DOPA to dopamine in extracerebral tissues 21,22. Carbidopa pretreatment improves imaging of the striatum by preventing early decarboxylation of 18F-DOPA to 18F-dopamine outside the brain. This will increase striatal uptake by increasing the concentration of 18F-DOPA in the plasma and decreasing renal excretion 23. Carbidopa is also used to increase 18F-DOPA uptake by tumor cells in the imaging of NETs 23,24. Carbidopa premedication consists of an oral administration of 100–250 mg of carbidopa 1 h before acquisition.
Timmers et al. 25 reported that the sensitivity of 18F-DOPA PET for the localization of PGL is improved by premedication with carbidopa. Carbidopa enhances the uptake of 18F-DOPA by PGL lesions and allows better discrimination between them and physiological tracer uptake by normal surrounding tissues. Moreover, carbidopa-enhanced scanning revealed additional lesions in one-third of the patients 25. Markedly increased uptake of 18F-DOPA has been noted in the basal ganglia, lungs, myocardium, and liver after premedication with carbidopa, but not in the pancreas 25. Physiological excretion into bile ducts, the gallbladder, and the urogenital system continued to be seen after administration of carbidopa. Lopci et al. 26 evaluated the effect of premedication with carbidopa on five children with a history of neuroblastoma. On a visual basis, a clear reduction in the abdominal accumulation of 18F-DOPA was observed in all cases after carbidopa premedication both to the biliary structures and to the excretory system and was accompanied by a generalized increase in soft-tissue uptake, as well as increased uptake in the basal ganglia and liver. The authors concluded that premedication with carbidopa seems to influence the biodistribution of 18F-DOPA in the liver and pancreas in a manner similar to that reported in adults 26.
However, premedication with carbidopa does not benefit all 18F-DOPA PET indications. Carbidopa premedication should not be administered when insulinomas or β cell hyperplasia in adults is suspected, as the reduced uptake in the whole pancreas (both healthy and pathological) could hide the pathological area and lead to false-negative results 8. Carbidopa premedication may improve the diagnostic performance of 18F-DOPA PET in the evaluation of the striatum and of some NETs such as PGL and carcinoid tumors, but it is not recommended in the evaluation of pancreatic lesions 24,25.
Image interpretation
18F-DOPA imaging follows a very specific metabolic process and presents unspecific accumulation only to its excretory pathways. In normal tissues, 18F-DOPA has minimal uptake and therefore provides good lesion-to-background ratios.
Patients who are referred for 18F-DOPA PET/CT already have a clinical suspicion of disease based on their clinical and/or biochemical findings or imaging results; hence, they present with a very precise clinical indication. Thus, it is helpful to be aware of what we expect to find (physiological pattern and variants), what we are looking for (a PGL rather than a PCC, a pulmonary carcinoid, a gastroenteropancreatic tumours NET, etc), its location (as locoregional relapse or in lymph nodes for MTC, in adrenals for suspected PCC, in the pancreas for insulinoma), its appearance (usually focal intense uptake that does not follow the physiological biodistribution), and what could mask its identification (uptake in excretory organs such as the gallbladder, pancreas, or urinary tract). Hybrid PET/CT systems have contributed to the introduction of another tool for the correct interpretation of areas of uptake, as we can rely on the CT images for anatomical details that can help localize the sites of 18F-DOPA uptake.
Variable 18F-DOPA uptake can be seen in the pancreas, especially in the uncinate process, which, in some cases, can show very intense uptake. Similarly, uptake by the adrenal glands may be highly variable and this must be taken into consideration to avoid misinterpretation of a normal adrenal as a PCC.
When the adrenal uptake is high but homogenous, symmetrical, and not associated with morphological alteration on CT imaging, it should be considered as indicating normal appearance. No significant or only mildly diffuse uptake is usually noted in the bowel. As already mentioned, the excretory organs (gallbladder, kidneys, and urinary bladder) show a high variability of SUVmax depending on the individual elimination timing and hydration status. The biodistribution data are relatively constant in terms of the intensity of liver uptake and could be helpful when semiquantitative analyses based on the lesion-to-background ratio are needed. For this purpose, the liver uptake could be regarded as a background parameter.
Areas of focal uptake outside the physiological distribution of the tracer can be considered pathological. In suspected PGL/PCC, any nonphysiological extra-adrenal focal uptake or asymmetrical adrenal uptake with a concordant enlarged gland or adrenal uptake more intense than that of the liver should be considered pathological 17.
In brain tumors the qualitative criterion used is that any tracer activity above the background level of the adjacent brain can be considered abnormal. Standard visual analysis of 18F-DOPA PET seems adequate with a high sensitivity in identifying the tumor. However, the specificity is low because all radiation necrosis lesions have low but visible tracer uptake 3. Therefore, different quantitative methods based on ratios between the tumor and the normal contralateral hemisphere (T/N), striatum (T/S), or white matter (T/W) can provide additional help. The specificity of 18F-DOPA in brain tumor imaging can be greatly increased using a T/S threshold of 0.75 or 1.0, a T/N threshold of 1.3, or a T/W threshold of 1.6 3. Using ROC curves, the optimal threshold for 18F-DOPA is the T/S ratio of greater than 1.0, which shows a sensitivity of 98%, a specificity of 86%, a PPV of 95%, and an NPV of 95%. A recent study demonstrated that uptake is significantly higher in high-grade tumors than in low-grade tumors in newly diagnosed (but not in recurrent) tumors, and an SUVmax of 2.72 could discriminate between low-grade and high-grade tumors, with a sensitivity and specificity of 85 and 89%, respectively 27. In the brain the only uptake of tracer is seen in the striatum, which makes interpretation difficult.
As already cited above, premedication with carbidopa enhances the uptake of 18F-DOPA by PGL lesions and significantly blocks physiological tracer uptake by the pancreas, which can be a potential confounder in the detection of adrenal lesions.
Possible pitfalls
Pitfalls related to the interpretation of PET and PET/CT images
Keeping in mind the physiological biodistribution of 18F-DOPA, any focal uptake of the tracer outside areas of normal uptake can be considered pathological in relation to the clinical suspicion.
Gallbladder/common bile tract
One of the major pitfalls of 18F-DOPA PET/CT scans could be the intense focal uptake of the tracer in the gallbladder and in some cases in the common bile tract, which could mimic an intestinal tumor or a hepatic metastasis by a neuroendocrine primary tumor 28. In this case, knowledge of the normal biodistribution of the tracer and its physiological excretion, coupled with correlative CT images of the PET/CT, can easily help identify the site of uptake as physiological activity in the gallbladder or the biliary path.
Urinary tract
Urinary excretion is the major excretory route of the tracer and it can be the cause of several pitfalls. The intense uptake of the tracer in the kidneys could ‘mask’ pathological uptake in the tail of the pancreas (left kidney), whereas activity in the right kidney interferes less with the head of the pancreas. Moreover, uptake in the kidneys could hide a pathological uptake of the adrenals, especially in patients with dilatation of the superior calyces. Uptake in the ureters, even if less intense and with a ‘spotting’ appearance, could resemble pathological abdominal uptake in the bowel or in lymph nodes. The bladder is less interfering as scanning usually starts with an empty bladder. In all cases the CT component of the hybrid PET/CT scanners is extremely helpful in localizing the anatomical counterpart of the uptake. Finally, late images after diuretic administration or after ambulation and hydration could alter the appearance of the uptake and help discriminate between pathologic and physiologic uptake.
Pancreas
An obvious limitation of pancreatic PET imaging using 18F-DOPA is the physiological uptake of the tracer in pancreatic tissue. The physiological intense and very variable uptake in the pancreas can lead to two possible pitfalls: on the one hand, uptake in the pancreas, especially in the uncinate process, can be confused as a para-aortic pathologic lesion (false positive); on the other, a pancreatic lesion with uptake similar to that of the rest of the gland may not be identified as pathological by 18F-DOPA (false negative). In addition, physiological pancreatic uptake is a potential limitation of 18F-DOPA PET in the detection of adrenal lesions, and in these cases premedication with carbidopa prevents masking of a possible lesion by blocking the pancreatic uptake. Carbidopa may also increase the uptake in the lesions to render it more easily identifiable.
The utility of 18F-DOPA PET/CT in adult patients with hyperinsulinemic hypoglycemia remains debatable, as the minor difference between pathological and nonpathological areas of the pancreas may present a possible pitfall. Premedication with carbidopa could lead to another possible methodological pitfall in hyperinsulinemic hypoglycemia by decreasing the entire pancreatic uptake and reducing the lesion-to-background ratio 25. Reversal of intense focal pancreatic uptake has been reported after premedication with carbidopa in patients with hyperinsulinemic hypoglycemia 29–32.
Pitfalls related to the pathology
Some possible sources of false-negative results of 18F-DOPA PET/CT can be related to factors such as the small size of the lesion or tumor dedifferentiation. Genetic factors may also affect the 18F-DOPA uptake in PG; SDHB gene mutations may result in extra-adrenal PG, for which 18F-DOPA PET shows a lower sensitivity than that for non-SDHB-related lesions 16. In contrast, the high specificity of 18F-DOPA PET and PET/CT, explained by the fact that only neuroendocrine cells are able to take up, decarboxylate, and store amino acids and their amines, leads to few false-positive 18F-DOPA PET findings.
Kauhanen et al. 2 described one patient with suspected PG recurrence and increased 18F-DOPA uptake in the right adrenal; histological verification showed a normal adrenal gland. Timmers et al. 33 reported one patient with a gastrointestinal stromal tumor that was visualized by 18F-DOPA PET. Luster et al. 34 described an adrenal mass with a mildly intense 18F-DOPA uptake, but clinical follow-up revealed no evidence of PCC. However, when an adrenal mass presents with high or very high radiotracer uptake a diagnosis of PCC is certain.
Rischke et al. 35 reported four patients with false-positive results (among 33 patients studied) with 18F-DOPA accumulation not specific for PCC or PGL; these results were verified by cross-sectional imaging and follow-up and revealed (i) an anatomic variant of pancreas morphology, (ii) diverticulum of the duodenum, (iii) moderate focal accumulation in paraesophageal tissue without evidence of a nodular structure, and (iv) moderate focal accumulation in paracolic tissue without evidence of a nodular structure 35.
Koopmans et al. 24 prospectively studied 53 patients with carcinoid tumor and they recorded a patient-based, region-based, and lesion-based sensitivity of 100, 95, and 96%, respectively, which was better than those of CT, somatostatin receptor scientigraphy, and combined CT/somatostatin receptor scientigraphy, with no false-positive cases.
A recent meta-analysis on MTC reported that false-positive findings with 18F-DOPA are uncommon; in contrast, false-negative results could be probably related to small MTC lesions or to dedifferentiation. In fact, comparative analysis between 18F-DOPA and 18F-FDG has shown better results for 18F-DOPA in terms of sensitivity and specificity. On the basis of findings in the literature, the diagnostic performance of 18F-DOPA in recurrent MTC improved in patients with higher serum calcitonin levels 36,37. High levels of calcitonin and negative 18F-DOPA PET could be due to a small recurrent tumor size. High levels of carcinoembryonic antigen and negative 18F-DOPA PET could result from dedifferentiation of the tumor and its inability to take up DOPA; hence, 18F-FDG would be the radiopharmaceutical of choice.
In a paper by Tessonier et al. 29 the authors report intense radiotracer uptake in the whole pancreas that was not statistically significant between controls (n=37) and hyperinsulinemic patients (n=6), with mean pancreatic SUVmax of 2.7 and 1.9, respectively; they concluded that 18F-DOPA PET/CT appears to have a limited role in tumor localization in hyperinsulinemic hypoglycemia patients. In contrast, Kauhanen et al. 4 on the same topic reported excellent results in nine of 10 patients with mean SUVmax in the affected pancreas of 4.4 compared with a mean SUVmax of 3.2 in other parts of the pancreas.
Other pitfalls of 18F-DOPA PET in PGL imaging include tracer accumulation in the gallbladder and renal collecting system, mimicking an extra-adrenal tumor 25. False-positive results may be related to tracer uptake by other neuroendocrine lesions. Occasionally, uptake may be due to nonspecific inflammation (pneumonia or postoperative changes), as high levels of amino acid transports have also been found in macrophages.
In the brain, the list of nontumoral areas and processes showing uptake of all radiolabeled amino acids is also long and includes ischemic brain areas, infarction, scar tissue, abscess, sarcoidosis, irradiated areas, hemangioma, and many other non-neoplastic processes. Active inflammatory cells also require amino acids, and the increased perfusion of infections may contribute even further to uptake of amino acids.
‘Technical’ pitfalls
PET/CT represents a major technological advancement, consisting of two complementary modalities that provide both functional and anatomic information and whose combined strength tends to overcome their respective weaknesses. With combined PET/CT, the superimposition of the precise structural findings provided by CT allows for an accurate correlation of the radiotracer activity seen at PET with the correct anatomic or pathologic equivalent. When attenuation correction is based on CT images there is a potential risk of overestimating the true activity of the tracer, such as in cases of photopenic areas corresponding to high-density structures on CT (metallic implants, surgical clips, barium) 38.
Another possible pitfall can be caused by misregistration between PET and CT images; thus, a superimposition of radiotracer activity on the wrong anatomic structure seen on CT images, which can be due to breathing, patient motion, or bowel motility, can lead to false-positive or false-negative PET findings.
Conclusion
18F-DOPA is a radiopharmaceutical with very interesting clinical applications and very promising performances in the evaluation of the integrity of dopaminergic pathways, brain tumors, NETs (especially MTC, PGL, and PCC), and congenital hyperinsulinism. 18F-DOPA traces a very specific metabolic pathway and has a very precise biodistribution pattern. As for any radiopharmaceutical, knowledge of the normal distribution of 18F-DOPA, its physiological variants, and possible pitfalls is essential for the correct interpretation of PET scans. Moreover, it is very important to be aware of the potential false-positive and false-negative cases that can occur in various clinical settings.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
Footnotes
Correspondence to Domenico Rubello, MD, Department of Imaging, Service of Nuclear Medicine, PET/CT Centre, Santa Maria della Misericordia Hospital, Via Tre Martiri 140, Rovigo 45100, Italy Tel: +39 0425 39 4428; fax: +39 0425 39 4434; e-mail: domenico.rubello@libero.it
References
- 1.Nanni C, Fantini L, Nicolini S, Fanti S.Non FDG PET.Clin Radiol 2010;65:536–548 [DOI] [PubMed] [Google Scholar]
- 2.Kauhanen S, Seppänen M, Ovaska J, Minn H, Bergman J, Korsoff P, et al. The clinical value of [18F]fluoro-dihydroxyphenylalanine positron emission tomography in primary diagnosis, staging, and restaging of neuroendocrine tumors.Endocr Relat Cancer 2009;16:255–265 [DOI] [PubMed] [Google Scholar]
- 3.Chen W.Clinical applications of PET in brain tumors.J Nucl Med 2007;48:1468–1481 [DOI] [PubMed] [Google Scholar]
- 4.Kauhanen S, Seppänen M, Minn H, Gullichsen R, Salonen A, Alanen K, et al. Fluorine-18-l-dihydroxyphenylalanine (18F-DOPA) positron emission tomography as a tool to localize an insulinoma or beta-cell hyperplasia in adult patients.J Clin Endocrinol Metab 2007;92:1237–1244 [DOI] [PubMed] [Google Scholar]
- 5.Mohnike K, Blakenstein O, Minn H, Mohnike W, Fuchtner F, Otonkoski T.[18F]-DOPA positron emission tomography for preoperative localization incongenital hyperinsulinism.Horm Res 2008;70:65–72 [DOI] [PubMed] [Google Scholar]
- 6.Caroli P, Nanni C, Rubello D, Alavi A, Fanti S.Non-FDG PET in the practice of oncology.Indian J Cancer 2010;47:120–125 [DOI] [PubMed] [Google Scholar]
- 7.Kao CHK, Hsu WL, Xie HL, Lin MC, Lan WC, Chao HY.GMP production of [18F]FDOPA and issue concerning its quality analyses as in USP ‘Fluorodopa F 18 Injection’.Ann Nucl Med 2011;25:309–316 [DOI] [PubMed] [Google Scholar]
- 8.Jager PL, Chirakal R, Marriott CJ, Brouwers AH, Koopmans KP, Gulenchyn KY.6-l-18F-Fluorodihydroxyphenylalanine PET in neuroendocrine tumors: basic aspects and emerging clinical applications.J Nucl Med 2008;49:573–586 [DOI] [PubMed] [Google Scholar]
- 9. Fluorodopa F18 (systemic). Available at: http://www.drugs.com [Accessed 25 August 2013]
- 10.Luxen A, Guillaume M, Melega WP, Pike VW, Solin O, Wagner R.Production of 6-[18F]fluoro-l-DOPA and its metabolism in vivo: critical review.Int J Rad Appl Instrum B 1992;19:149–158 [DOI] [PubMed] [Google Scholar]
- 11.Mohnike K, Blankenstein O, Christesen HT, De Lonlay J, Hussain K, Koopmans KP, et al. Proposal for a standardized protocol for 18F-DOPA-PET (PET/CT) in congenital hyperinsulinism.Horm Res 2006;66:40–42 [DOI] [PubMed] [Google Scholar]
- 12.Grassetto G, Massaro A, Cittadin S, Marzola MC, Chondrogiannis C, Rampin L, Rubello D.Kinetic of 18F-DOPA in basal ganglia of non-human primate.Eur J Nucl Med Mol Imaging 2009;Suppl 2:145–539 [Google Scholar]
- 13.Koopmans KP, Brouwers AH, De Hooge MN, Van der Horst-Schrivers AN, Kema IP, Wolffenbuttel BH, et al. Carcinoid crisis after injection of 6-18F-fluorodihydroxyphenylalanine in a patient with metastatic carcinoid.J Nucl Med 2005;46:1240–1243 [PubMed] [Google Scholar]
- 14.Schiesser M, Veit-Haibach P, Muller MK, Weber M, Bauerfeind P, Hany T, Clavien PA.Value of combined 6-[18F]fluorodihydroxyphenylalanine PET/CT for imaging of neuroendocrine tumours.Br J Surg 2010;97:691–697 [DOI] [PubMed] [Google Scholar]
- 15.Treglia G, Rufini V, Salvatori M, Giordano A, Giovanella L.PET imaging in recurrent medullary thyroid carcinoma.Int J Mol Imaging 2012;2012:324686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Treglia G, Cocciolillo F, de Waure C, Di Nardo F, Gualano MR, Castaldi P, et al. Diagnostic performance of 18F-dihydroxyphenylalanine positron emission tomography in patients with paraganglioma: a meta-analysis.Eur J Nucl Med Mol Imaging 2012;39:1144–1153 [DOI] [PubMed] [Google Scholar]
- 17.Taïeb D, Timmers HJ, Hindié E, Guillet BA, Neumann HP, Walz MK, et al. EANM 2012 guidelines for radionuclide imaging of phaeochromocytoma and paraganglioma.Eur J Nucl Med Mol Imaging 2012;39:1977–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beheshti M, Pocher S, Vali R, Waldenberger P, Broinger G, Nader M, et al. The value of 18F-DOPA PET-CT in patients with medullary thyroid carcinoma: comparison with 18F-FDG PET-CT.Eur Radiol 2009;19:1425–1434 [DOI] [PubMed] [Google Scholar]
- 19.Soussan M, Nataf V, Kerrou K, Grahek D, Pascal O, Talbot JN, et al. Added value of early 18F-FDOPA PET/CT acquisition time in medullary thyroid cancer.Nucl Med Commun 2012;33:775–779 [DOI] [PubMed] [Google Scholar]
- 20.Chondrogiannis S, Grassetto G, Marzola MC, Rampin L, Massaro A, Bellan E, et al. 18F-DOPA PET/CT biodistribution consideration in 107 consecutive patients with neuroendocrine tumours.Nucl Med Commun 2012;33:179–184 [DOI] [PubMed] [Google Scholar]
- 21.Jaffe M.Clinical studies of carbidopa and l-DOPA in the treatment of Parkinson’s disease.Adv Neurol 1973;2:161 [Google Scholar]
- 22.Pinder RM, Brogden RN, Sawyer PR, Speight TM, Avery GS.Levodopa and decarboxylase inhibitors: a review of their clinical pharmacology and use in the treatment of parkinsonism.Drugs 1976;11:329–377 [DOI] [PubMed] [Google Scholar]
- 23.Brown WD, Oakes TR, DeJesus OT, Taylor MD, Roberts dC, Nickles RJ, Holden JE.Fluorine-18-fluoro-l-DOPA dosimetry with carbidopa pretreatment.J Nucl Med 1998;39:1884–1891 [PubMed] [Google Scholar]
- 24.Koopmans KP, de Vries EG, Kema IP, Elsinga PH, Neels OC, Sluiter WJ, et al. Staging of carcinoid tumours with 18F-DOPA PET: a prospective, diagnostic accuracy study.Lancet Oncol 2006;7:728–734 [DOI] [PubMed] [Google Scholar]
- 25.Timmers HJ, Hadi M, Carrasquillo JA, Chen CC, Martiniova L, Whatley M, et al. The effects of carbidopa on uptake of 6-18F-fluoro-l-DOPA in PET of pheochromocytoma and extraadrenal abdominal paraganglioma.J Nucl Med 2007;48:1599–1606 [DOI] [PubMed] [Google Scholar]
- 26.Lopci E, D’Ambrosio D, Nanni C, Chiti A, Pession A, Marengo M, Fanti S.Feasibility of carbidopa premedication in pediatric patients: a pilot study.Cancer Biother Radiopharm 2012;27:729–733 [DOI] [PubMed] [Google Scholar]
- 27.Fueger BJ, Czernin J, Cloughesy T, Silverman DH, Geist CL, Walter MA, et al. Correlation of 6-18F-fluoro-l-dopa PET uptake with proliferation and tumor grade in newly diagnosed and recurrent gliomas.J Nucl Med 2010;51:1532–1538 [DOI] [PubMed] [Google Scholar]
- 28.Balan KK.Visualization of the gall bladder on F-18 FDOPA PET imaging: a potential pitfall.Clin Nucl Med 2005;30:23–24 [DOI] [PubMed] [Google Scholar]
- 29.Tessonnier L, Sebag F, Ghander C, De Micco C, Reynaud R, Palazzo FF, et al. Limited value of 18F-F-DOPA PET to localize pancreatic insulin-secreting tumors in adults with hyperinsulinemic hypoglycemia.J Clin Endocrinol Metab 2010;95:303–307 [DOI] [PubMed] [Google Scholar]
- 30.de Lonlay P, Simon-Carre A, Ribeiro MJ, Boddaert N, Giurgea IO, Laborde K, et al. Congenital hyperinsulinism: pancreatic [18F]fluoro-l-dihydroxyphenylalanine (DOPA) positron emission tomography and immunohistochemistry study of DOPA decarboxylase and insulin secretion.J Clin Endocrinol Metab 2006;91:933–940 [DOI] [PubMed] [Google Scholar]
- 31.Ribeiro MJ, De Lonlay P, Delzescaux T, Boddaert N, Jaubert F, Bourgeois S, et al. Characterization of hyperinsulinism in infancy assessed with PET and 18F-fluoro-l-DOPA.J Nucl Med 2005;46:560–566 [PubMed] [Google Scholar]
- 32.Kauhanen S, Seppänen M, Nuutila P.Premedication with carbidopa masks positive finding of insulinoma and β-cell hyperplasia in [(18)F]-dihydroxy-phenyl-alanine positron emission tomography.J Clin Oncol 2008;26:5307–5308author reply 5308–5309 [DOI] [PubMed] [Google Scholar]
- 33.Timmers HJ, Chen CC, Carrasquillo JA, Whatley M, Ling A, Havekes B, et al. Comparison of 18F-fluoro-l-DOPA, 18F-fluoro-deoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma.J Clin Endocrinol Metab 2009;94:4757–4767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luster M, Karges W, Zeich K, Pauls S, Verburg FA, Dralle H, et al. Clinical value of 18F-fluorodihydroxyphenylalanine positron emission tomography/computed tomography (18F-DOPA PET/CT) for detecting pheochromocytoma.Eur J Nucl Med Mol Imaging 2010;37:484–493 [DOI] [PubMed] [Google Scholar]
- 35.Rischke HC, Benz MR, Selvaggio D, Mix M, Dumont RA, Campbell D, et al. Correlation of the genotype of paragangliomas and pheochromocytomas with their metabolic phenotype on 3,4-dihydroxy-6-18F-fluoro-l-phenylalanin PET.J Nucl Med 2012;53:1352–1358 [DOI] [PubMed] [Google Scholar]
- 36.Treglia G, Cocciolillo F, Di Nardo F, Poscia A, de Waure C, Giordano A, Rufini V.Detection rate of recurrent medullary thyroid carcinoma using fluorine-18 dihydroxyphenylalanine positron emission tomography: a meta-analysis.Acad Radiol 2012;19:1290–1299 [DOI] [PubMed] [Google Scholar]
- 37.Marzola MC, Pelizzo MR, Ferdeghini M, Toniato A, Massaro A, Ambrosini V, et al. Dual PET/CT with (18)F-DOPA and (18)F-FDG in metastatic medullary thyroid carcinoma and rapidly increasing calcitonin levels: comparison with conventional imaging.Eur J Surg Oncol 2010;36:414–421 [DOI] [PubMed] [Google Scholar]
- 38.Blake MA, Singh A, Setty BN, Slattery J, Kalra M, Maher MM, et al. Pearls and pitfalls in interpretation of abdominal and pelvic PET-CT.Radiographics 2006;26:1335–1353 [DOI] [PubMed] [Google Scholar]


