FIG. 1.
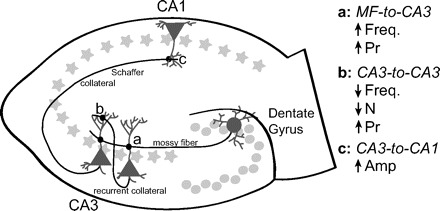
Homeostatic adaptations at tetrodotoxin (TTX)-treated hippocampal synapses maintain network stability. The hippocampal circuit is primarily viewed as a relatively simplistic feedforward excitatory pathway. Information from the entorhinal cortex travels along the perforant pathway to dentate gyrus granule (DG) cells, which send mossy fibers (MFs) to CA3 cells. From here, information flows from CA3 cells to CA1 cells via Schaffer collaterals. Following chronic TTX application to suppress spiking two types of hippocampal throughput synapses, MF-to-CA3 and CA3-to-CA1, show an increase in activity. Such increases in activity following activity deprivation are an expected homeostatic adaptation. At MF-to-CA3 synapses, the frequency (Freq) of miniature excitatory postsynaptic currents (mEPSCs) and the probability of presynaptic neurotransmitter release (Pr) both increase. mEPSC amplitude (Amp) is up-regulated at CA3-to-CA1 synapses. On the other hand, CA3-to-CA3 synapses show a decrease in activity, an unexpected homeostatic adaptation to inactivity. At these synapses, the increase in probability of presynaptic transmitter release is counterbalanced by a decrease in the number of active postsynaptic sites (N) responding to transmitter release. This synaptic silencing is primarily responsible for the decrease in mEPSC frequency that is also observed at these synapses. All of these adaptations could function in concert to make the network more stable by controlling reverberatory bursting, thereby guarding the network against harmful runaway excitation underlying epileptogenic activity.
