Abstract
Arbuscular mycorrhizal fungi (AMF), which are present in most natural environments, have demonstrated capacity to promote biodegradation of organic pollutants in the greenhouse. However, it is not certain whether AMF can spontaneously establish in phytoremediation systems constructed to decontaminate groundwater, because of the unusual conditions during the construction and operation of such systems. To assess this possibility, root samples from a wetland constructed for the phytoremediation of groundwater contaminated with benzene, methyl tert-butyl ether and ammonia were analysed. Substantial AMF colonization was observed in plant roots sampled close to the inlet of a basin filled with fine gravel and planted with Phragmites australis. In addition, analysis of a fragment of the nuclear large ribosomal subunit, amplified by nested PCR, revealed the presence of AMF molecular operational taxonomic units closely related to Funneliformis mosseae and Rhizophagus irregularis in the samples. These findings demonstrate the capacity of generalist AMF strains to establish spontaneously, rapidly and extensively in groundwater bioremediation technical installations.
Introduction
An arbuscular mycorrhiza is a type of close, mutualistic association that forms in root systems between diverse plant species and members of a small group of soil fungi (arbuscular mycorrhizal fungi, AMF; Smith and Read, 2008). The association allows the exchange of nutrients (carbohydrates provided by the plant, mineral nutrients provided by the fungi), and markedly increases the host plant's tolerance of various biotic and abiotic stress factors. Arbuscular mycorrhizal fungi also influence the transport and distribution of organic pollutants in plants (Debiane et al., 2009; Langer et al., 2010), reportedly reducing their concentrations in shoots of colonized plants, while increasing their concentrations in roots, particularly in the rhizodermis (Huang et al., 2007; Wu et al., 2009). These effects may help to protect plants from damage by organic pollutants. Beneficial effects of the presence of AMF on soil bacteria (Toljander et al., 2007), notably bacteria capable of degrading organic compounds (Corgié et al., 2006; Alarcon et al., 2008), have also been reported. By both protecting plants from adverse effects of organic pollutants and promoting associated bacteria, AMF can accelerate the biodegradation of organic pollutants. Several studies have recently demonstrated beneficial effects of AMF on the biodegradation of organic pollutants, including: the dissipation of polycyclic aromatic hydrocarbons (PAHs) by Lolium multiflorum (Yu et al., 2011), dissipation of PAHs by Medicago sativa under low water and phosphate availability (Zhou et al., 2009), and phytoremediation of aged petroleum contamination by Triticum aestivum (Malachowska-Jutsz and Kalka, 2010). Arbuscular mycorrhizal fungi can therefore be considered ideal inhabitants of technical installations for the plant-based bioremediation of groundwater contaminated by organic pollutants. However, such installations are often constructed without including a significant source of AMF propagules. Furthermore, the stressful conditions in such installations – such as poor substrates, and potentially toxic concentrations of organic pollutants for the fungi (Verdin et al., 2006; Debiane et al., 2011) – may hinder the successful establishment of AMF.
To investigate the ability of AMF to establish under such conditions, we analysed AMF colonization levels in plant roots sampled from a wetland constructed to decontaminate groundwater polluted with benzene, methyl tert-butyl ether (MTBE) and ammonia. The wetland was continuously streamed (inflow rate 6 l h−1) by water containing 20, 3.7 and 45 mg l−1 of these compounds respectively. Arbuscular mycorrhizal fungi present in roots from Phragmites australis growing in this wetland were phylogenetically analysed by cloning and sequencing a 400 bp fragment of the nuclear large ribosomal subunit, amplified by nested PCR.
Results and discussion
Spontaneous colonization of constructed wetlands
The constructed wetland investigated in this study was established in March 2007. It consists of a basin that receives a stream of contaminated groundwater. Phragmites australis plantlets were planted at the inlet end, which is filled with light gravel. Close to its outlet area there is a compartment lacking the gravel substrate where P. australis is growing in water, forming a dense root mat (Fig. 1). Root samples taken from the part of the constructed wetland with the gravel substrate in 2011 were substantially associated with AMF (colonized proportions by length, 40%, 25%, 25%, 60% and 80%; see Fig. 1 legend for details), clearly showing that these fungi successfully colonized this unusual environment within 4 years. Thus, establishment of AMF does not appear to have been profoundly hindered in the inlet part of the wetland, although it was exposed to the highest concentrations of organic pollutants. In contrast, no colonization of roots by AMF was observed in the part of the basin where the plants were growing in free water with no gravel substrate, suggesting that a solid substrate was required for AMF colonization. The likeliest sources of the colonizing fungi were airborne propagules or mycelia already present in the P. australis plantlets when they were transferred to the constructed wetland.
Fig. 1.
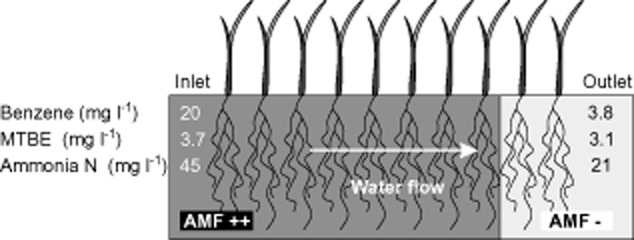
In March 2011 five samples of roots (each about 10 g) were taken from the ‘front’ (near the inlet) and five from the ‘rear’ (near the outlet; 10 samples in total) of the illustrated constructed wetland (5 m long, 1.15 m wide, 1.25 m deep; inflow rate 6 l h−1) planted with P. australis, which is being used in a compartment transfer experiment close to Leuna, Germany (Seeger et al., 2011). Parts of the sampled roots were stained with ink (Sheaffer, Middlesex, UK) and vinegar according to Vierheilig and colleagues (1998) to highlight AMF structures, and the degree of colonization by AMF was roughly estimated by inspecting the stained roots under a stereomicroscope and estimating approximate ratios of mycorrhizally colonized to non-colonized root lengths. Substantial degrees of AMF colonization were observed in all five root samples from the ‘front’ part of the wetland (40%, 25%, 25%, 60% and 80%). In contrast, no colonization of P. australis roots was observed in samples from the rear part, where there was no gravel substrate and the roots formed a dense root mat. These microscopic observations are consistent with results of nested PCR analysis of a 400 bp fragment of the nuclear large ribosomal subunit using the primer pairs LR1/FLR2 and FLR3/FLR4 (Gollotte et al., 2004) and Taq PCR Mastermix (Qiagen, Hilden, Germany). DNA extracted (using a DNeasy Plant Mini-Kit, Qiagen) from all samples from the front part of the wetland yielded fragments of expected size (for AMF), while DNA extracted from samples from the rear part yielded no PCR products. The concentrations of pollutants (benzene, methyl tert-butyl ether/MTBE and ammonia N) shown in the figure have been taken from Seeger and colleagues (2011).
Generalist AMF strains as early and rapid colonizers of the constructed wetland
Considerable frequencies of very similar patterns were detected in restriction fingerprinting of PCR products cloned from a fragment of the large ribosomal subunit, indicating that the AMF community within the constructed wetland had low diversity at the sampling time. Fifty-one clones with identical patterns were removed from the analysis, leaving 34 unique clones for sequence analysis, and only two AMF taxa were detected: Rhizophagus irregularis and Funneliformis mosseae. The restriction endonuclease Taq I was used for restriction fingerprinting, partly because it has been recommended for T-RFLP analysis of the PCR fragment analysed in this study (Mummey and Rillig, 2007), and partly because almost all AMF species in the phylogenetic tree shown in Fig. 2 could be differentiated using this enzyme in a virtual digest. In particular, it was possible to differentiate all other species from R. irregularis and F. mosseae, the two AMF found in the wetland samples, excluding the possibility that AMF species were missed because of the use of Taq I for restriction fingerprinting prior to sequence analysis. As the primer pairs used in our analysis are not capable of amplifying sequences of members from the genus Diversispora or the families Archaeosporaceae and Paraglomaceae (Gamper et al., 2009), however, the possible presence of additional AMF from these groups cannot be excluded. Phylogenetic analysis using the set of consensus sequences for AMF (see fig. 1 in Krüger et al., 2011) clearly showed that all sequences analysed in our study clustered with the AMF genera Rhizophagus or Funneliformis (data not shown). Only four sequences clustered with different fungal groups, one of which proved to be a chimeric sequence in later analysis, while the other three were very similar to sequences of the basidiomycotan genus Cryptococcus. We have previously observed unspecific amplification of nuclear rRNA from this genus using the primers FLR3 and FLR4 on a number of occasions. Cryptococcus is a large fungal genus with some species that are pathogenic for humans. Although substrate (light gravel) and inflowing water (contaminated groundwater) can be expected to be relatively poor inocula in general, introduction of members from Cryptococcus by these sources cannot be excluded. Alternatively, airborne spores have been described for the pathogenic species (Hajjeh et al., 1995; Kidd et al., 2007) and appear also possible as sources of inoculation in the case presented here.
Fig. 2.
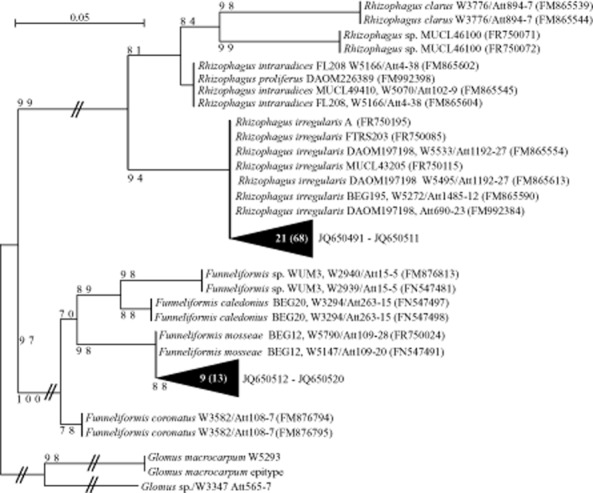
PCR amplification products of a fragment of the nuclear large ribosomal subunit obtained from DNA in root samples collected from the front part of the constructed wetland were purified (using a peqGold Cycle-Pure Kit; Peqlab, Erlangen, Germany), pooled and ligated into pCR 2.1 (using a TA cloning kit; Life Technologies, Darmstadt, Germany). After transformation into Escherichia coli DH5α, 85 positive clones were identified using classical blue–white screening, and amplified by colony PCR (using M13 primers). The resulting products were screened by Taq I restriction digestion at 37°C overnight, and analysis of electrophoretic patterns using GelCompar II (Applied Maths NV, Sint-Marten-Latern, Belgium). Forty-eight clones with identical restriction patterns were identified and eliminated from further analysis. PCR products from the remaining 34 clones were purified using a SureClean kit from Bioline (Luckenwalde, Germany) and sequenced using a BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, USA) and a 3130xl Genetic Analyzer (Applied Biosystems). Sequences were edited (by removing primer and vector sequences, and controlling sequence quality) using Sequencher 4.8 (Gene Codes Corporation, USA). Database searches for similar sequences were performed using the blast program (Altschul et al., 1990). In a few cases, non-glomeromycotan sequences (similar to sequences from the basidiomycotan genus Cryptococcus) were found. The ClustalW2 algorithm implemented in Seaview (Gouy et al., 2010) was used to align sequences with corresponding sequences from AMF strains defined in Krüger and colleagues (2011). Most of these sequences refer to individual GenBank accessions, although the sequences for Glomus sp. W3347/Att565-7, Glomus macrocarpum W5293 and G. macrocarpum epitype refer to consensus sequences defined in Krüger and colleagues (2011). Seaview was also used to construct neighbour joining trees (using BioNJ and Kimura 2-parameter models, with 1000 bootstrap permutations) and the maximum likelihood tree shown here (model: general time reversible, starting from a neighbour joining/BioNJ tree, with branch support estimated using the approximate likelihood ratio test approach). The genus Glomus was used as an out-group in this tree. Branches were collapsed to those branches showing unique Taq I restriction patterns in a virtual digest. GenBank accession numbers for the sequences obtained in this study (black triangles) are shown. Numbers within the triangles refer to the numbers of respective sequences analysed and to numbers of clones with concordant Taq I digestion patterns (in brackets).
To examine the sequences clustering with Rhizophagus or Funneliformis in more detail, all reference sequences not belonging to either of these genera or the out-group genus Glomus were removed, while more sequences from Rhizophagus and Funneliformis – summarized by Krüger and colleagues (2011) – were included in the analysis. After sequence alignment and construction of a maximum likelihood tree (using the general time reversible evolutionary model), the sequence groups clustering with Funneliformis showed a close relationship with F. mosseae, while those clustering with Rhizophagus clustered exclusively with sequences from R. irregularis (Fig. 2). The phylogenetic tree produced from the applied sequences (Fig. 2) was therefore restricted to those branches in R. irregularis and F. mosseae that can be differentiated by virtual digestion using Taq I. As already mentioned, the possible presence of additional AMF from the Diversispora, Archaeosporaceae or Paraglomaceae cannot be excluded, because of limitations of the primers used in our analysis. Nevertheless, the observation only of sequences connected to R. irregularis and F. mosseae, after screening 85 and sequencing 34 sequences, corroborates the preliminary indications that the constructed wetland contained an AMF community with very low diversity.
Rhizophagus irregularis refers to a large part of the taxonomic group that was previously known as Glomus intraradices, while F. mosseae was previously named Glomus mosseae (Schüßler and Walker, 2010). Both of these species are known to be typical generalist AMF (Öpik et al., 2006; Rosendahl et al., 2009; Oehl et al., 2010) that have been found in diverse habitats around the world. Although they have not been mentioned specifically in analyses of AMF succession (Piotrowski and Rillig, 2008), they seem to be pioneer AMF strains in the constructed wetland we studied.
Acknowledgments
Elke Häusler provided excellent technical assistance. The constructed wetland examined was established and funded by the Helmholtz Centre for Environmental Research – UFZ within the scope of the SAFIRA II Research Programme (Revitalization of Contaminated Land and Groundwater at Megasites, subproject ‘Compartment Transfer – CoTra’). The manuscript has been reviewed by SEES Editing (Weston-Super-Mare, North Somerset, UK).
Conflict of interest
None declared.
References
- Alarcon A, Davies FT, Autenrieth RL, Zuberer DA. Arbuscular mycorrhiza and petroleum-degrading microorganisms enhance phytoremediation of petroleum-contaminated soil. Int J Phytoremediation. 2008;10:251–263. doi: 10.1080/15226510802096002. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Corgié SC, Fons F, Beguiristain T, Leyval C. Biodegradation of phenanthrene, spatial distribution of bacterial populations and dioxygenase expression in the mycorrhizosphere of Lolium perenne inoculated with Glomus mosseae. Mycorrhiza. 2006;16:207–212. doi: 10.1007/s00572-006-0049-6. [DOI] [PubMed] [Google Scholar]
- Debiane D, Garcon G, Verdin A, Fontaine J, Durand R, Shirali P, et al. Mycorrhization alleviates benzo[a]pyrene-induced oxidative stress in an in vitro chicory root model. Phytochemistry. 2009;70:1421–1427. doi: 10.1016/j.phytochem.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Debiane D, Calonne M, Fontaine J, Laruelle F, Grandmougin-Ferjani A, Sahraoui ALH. Lipid content disturbance in the arbuscular mycorrhizal, Glomus irregulare grown in monoxenic conditions under PAHs pollution. Fungal Biol. 2011;115:782–792. doi: 10.1016/j.funbio.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Gamper HA, Walker C, Schüßler A. Diversispora celata sp. nov: molecular ecology and phylotaxonomy of an inconspicuous arbuscular mycorrhizal fungus. New Phytol. 2009;182:495–506. doi: 10.1111/j.1469-8137.2008.02750.x. [DOI] [PubMed] [Google Scholar]
- Gollotte A, van Tuinen D, Atkinson D. Diversity of arbuscular mycorrhizal fungi colonising roots of the grass species Agrostis capillaris and Lolium perenne in a field experiment. Mycorrhiza. 2004;14:111–117. doi: 10.1007/s00572-003-0244-7. [DOI] [PubMed] [Google Scholar]
- Gouy M, Guindon S, Gascuel O. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol. 2010;27:221–224. doi: 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
- Hajjeh RA, Brandt ME, Pinner RW. Emergence of cryptococcal disease: epidemiologic perspectives 100 years after its discovery. Epidemiol Rev. 1995;17:303–320. doi: 10.1093/oxfordjournals.epirev.a036195. [DOI] [PubMed] [Google Scholar]
- Huang H, Zhang S, Shan XQ, Chen BD, Zhu YG, Nigel J, Bell B. Effect of arbuscular mycorrhizal fungus (Glomus caledonium) on the accumulation and metabolism of atrazine in maize (Zea mays L.) and atrazine dissipation in soil. Environ Pollut. 2007;146:452–457. doi: 10.1016/j.envpol.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Kidd SE, Chow Y, Mak S, Bach PJ, Chen H, Hingston AO, et al. Characterization of environmental sources of the human and animal pathogen Cryptococcus gattii in British Columbia, Canada, and the Pacific Northwest of the United States. Appl Environ Microbiol. 2007;73:1433–1443. doi: 10.1128/AEM.01330-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger M, Krüger C, Walker C, Stockinger H, Schüßler A. Phylogenetic reference data for systematics and phylotaxonomy of arbuscular mycorrhizal fungi from phylum to species level. New Phytol. 2011;193:970–984. doi: 10.1111/j.1469-8137.2011.03962.x. [DOI] [PubMed] [Google Scholar]
- Langer I, Syafruddin S, Steinkellner S, Puschenreiter M, Wenzel WW. Plant growth and root morphology of Phaseolus vulgaris L. grown in a split-root system is affected by heterogeneity of crude oil pollution and mycorrhizal colonization. Plant Soil. 2010;332:339–355. [Google Scholar]
- Malachowska-Jutsz A, Kalka J. Influence of mycorrhizal fungi on remediation of soil contaminated by petroleum hydrocarbons. Environ Bull. 2010;19:3217–3223. [Google Scholar]
- Mummey DL, Rillig MC. Evaluation of LSU rRNA-gene PCR primers for analysis of arbuscular mycorrhizal fungal communities via terminal restriction fragment length polymorphism analysis. J Microbiol Methods. 2007;70:200–204. doi: 10.1016/j.mimet.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Oehl F, Laczko E, Bogenrieder A, Stahr K, Bösch R, van der Heijden M, Sieverding E. Soil type and land use intensity determine the composition of arbuscular mycorrhizal fungal communities. Soil Biol Biochem. 2010;42:724–738. [Google Scholar]
- Öpik M, Moora M, Liira J, Zobel M. Composition of root-colonizing arbuscular mycorrhizal fungal communities in different ecosystems around the globe. J Ecol. 2006;94:778–790. [Google Scholar]
- Piotrowski JS, Rillig MC. Succession of arbuscular mycorrhizal fungi: patterns, causes, and considerations for organic agriculture. Adv Agron. 2008;97:111–130. [Google Scholar]
- Rosendahl S, McGee P, Morton JB. Lack of global population genetic differentiation in the arbuscular mycorrhizal fungus Glomus mosseae suggests a recent range expansion which may have coincided with the spread of agriculture. Mol Ecol. 2009;18:4316–4329. doi: 10.1111/j.1365-294X.2009.04359.x. [DOI] [PubMed] [Google Scholar]
- Schüßler A, Walker C. The Glomeromycota: A Species List with New Families and Genera. Edinburgh & Kew, UK: The Royal Botanic Garden; 2010. Munich, Germany: Botanische Staatssammlung Munich; Oregon, USA: Oregon State University. URL http://www.lrz.de/∼schuessler/amphylo/. ISBN-10: 1466388048. [Google Scholar]
- Seeger EM, Kuschk P, Fazekas H, Grathwohl P, Kaestner M. Bioremediation of benzene-, MTBE- and ammonia-contaminated groundwater with pilot-scale constructed wetlands. Environ Pollut. 2011;159:3769–3776. doi: 10.1016/j.envpol.2011.07.019. [DOI] [PubMed] [Google Scholar]
- Smith SE, Read D. Mycorrhizal Symbiosis. New York, NY, USA: Academic Press; 2008. [Google Scholar]
- Toljander JF, Lindahl BD, Paul LR, Elfstrand M, Finlay RD. Influence of arbuscular mycorrhizal mycelial exudates on soil bacterial growth and community structure. FEMS Microbiol Ecol. 2007;61:295–304. doi: 10.1111/j.1574-6941.2007.00337.x. [DOI] [PubMed] [Google Scholar]
- Verdin A, Sahraoui ALH, Fontaine J, Grandmougin-Ferjani A, Durand R. Effects of anthracene on development of an arbuscular mycorrhizal fungus and contribution of the symbiotic association to pollutant dissipation. Mycorrhiza. 2006;16:397–405. doi: 10.1007/s00572-006-0055-8. [DOI] [PubMed] [Google Scholar]
- Vierheilig H, Coughlan AP, Wyss U, Piché Y. Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Environ Microbiol. 1998;64:5004–5007. doi: 10.1128/aem.64.12.5004-5007.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N, Huang H, Zhang S, Zhu YG, Christie P, Zhang Y. Phenanthrene uptake by Medicago sativa L. under the influence of an arbuscular mycorrhizal fungus. Environ Pollut. 2009;157:1613–1618. doi: 10.1016/j.envpol.2008.12.022. [DOI] [PubMed] [Google Scholar]
- Yu XZ, Wu SC, Wu FY, Wong MH. Enhanced dissipation of PAHs from soil using mycorrhizal ryegrass and PAH-degrading bacteria. J Hazard Mater. 2011;186:1206–1217. doi: 10.1016/j.jhazmat.2010.11.116. [DOI] [PubMed] [Google Scholar]
- Zhou XB, Cébron A, Béguiristain T, Leyval C. Water and phosphorus content affect PAH dissipation in spiked soil planted with mycorrhizal alfalfa and tall fescue. Chemosphere. 2009;77:709–713. doi: 10.1016/j.chemosphere.2009.08.050. [DOI] [PubMed] [Google Scholar]


