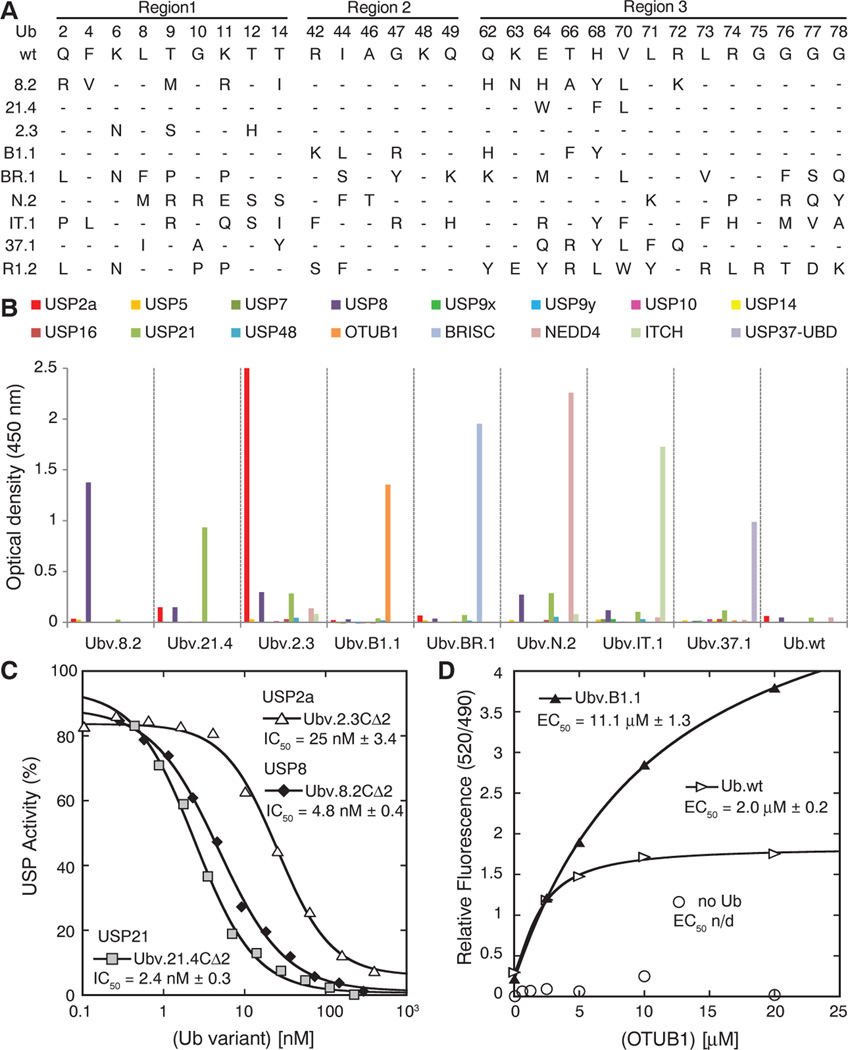
Fig. 2.
Selective binding of Ub variants to DUBs, Ub ligases, and UBDs, and inhibition of DUB function in vitro. (A) Sequence alignment of Ub.wt and representative Ub variants selected for binding to USP8 (8.2), USP21 (21.4), USP2a (2.3), OTUB1 (B1.1), the BRISC protein complex (BR.1), NEDD4 (N.2), ITCH (IT.1), USP37-UBD (37.1), or Cdc34 (R1.2). The alignment shows only those positions that were diversified in the Ub library, and positions that were conserved as the wt sequence are indicated by dashes. A complete list of selected variants is provided in table S2. (B) Ub variants bind selectively to their cognate targets, as shown by phage enzyme-linked immunosorbent assays for binding to the following immobilized proteins (color coded as indicated in the panel): USP2a, USP5, USP7, USP8, USP9x, USP9y, USP10, USP14, USP16, USP21, USP48, OTUB1, BRISC, NEDD4, ITCH, and USP37-UBD. Bound phage were detected spectrophotometrically (optical density at 450 nm), and background binding to neutravidin was subtracted from the signal. (C) Inhibition of USP2a, USP28, or USP21 shown as dose-response curves for Ub variants. The IC50 value was determined as the concentration of Ub variant that reduced USP activity by 50%. (D) The effects of Ubv.B1.1 or Ub. wt on binding of OTUB1 to Ub~UbcH5B measured as relative fluorescence by means of time-resolved Förster-energy transfer. The median effective concentration (EC50) value is defined as the half-maximal effective concentration of OTUB1.