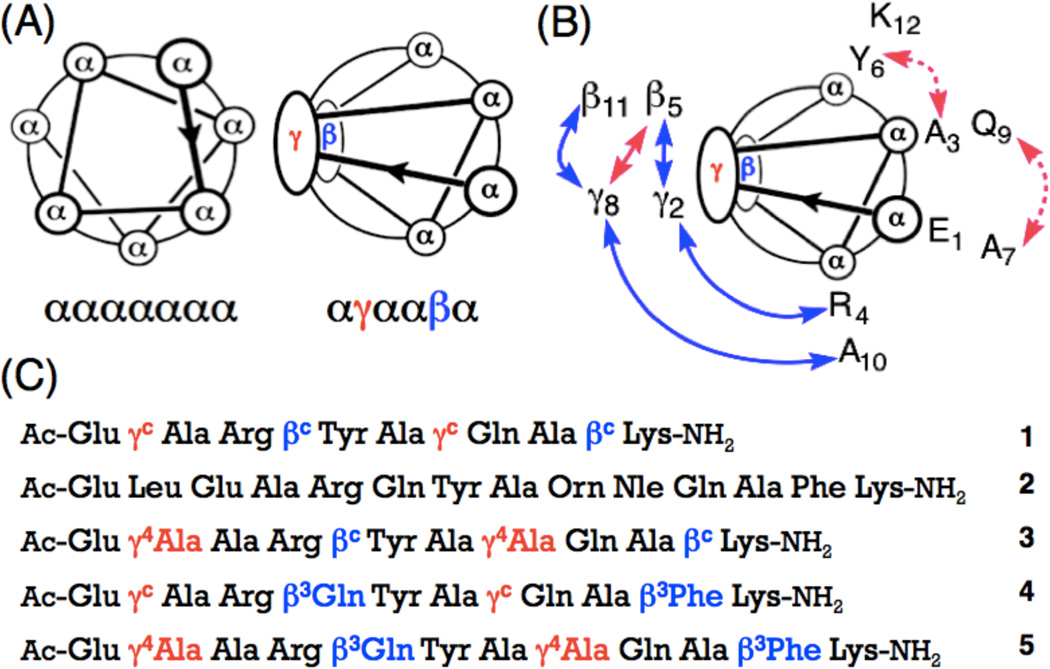
Figure 1.
(a) Helical wheel diagrams for the ααααααα heptad and the αγααβα hexad. (b) NOEs observed between nonadjacent residues for α/β/γ-peptide 3 in CD3OH (blue and pink, peptide concentration 6 mM), or in aqueous condition (blue, peptide concentration 2 mM) at 10 °C. Medium (2.6–3.5 Å) and weak (3.6–5.5 Å) NOEs are shown as curved arrows on the helical wheel diagram, (c) Sequences of α/β/γ-peptides 1, 3–5 and α-peptide 2. Superscript ‘c’ indicates a ring-constrained residue; the structures of βc and γc are shown in Figure 2 (All stereocenters are S).