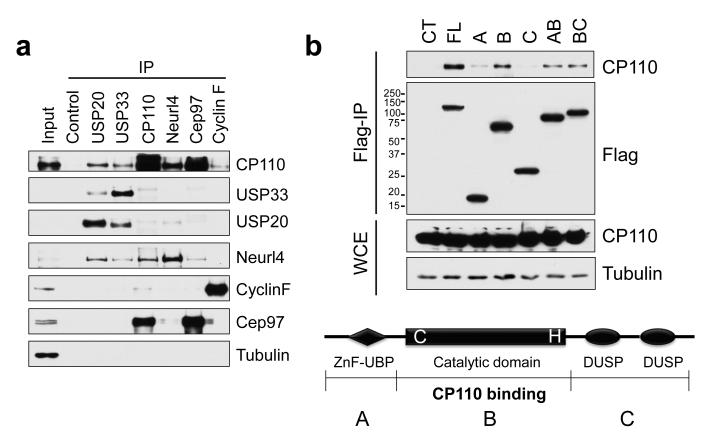
Figure 1. Identification of USP33 as a CP110-associated protein.
(a) HEK293T cell extracts were immunoprecipitated with the indicated antibodies (top), and western blots were probed as shown (right). Non-specific rabbit IgG was used as control. α-tubulin is shown as a negative control. (b) (Bottom) Domains of USP33 are indicated. The catalytic domain of USP33 stably binds to CP110. Double cysteine and histidine point mutations in the catalytic domain (DM mutant) render the DUB inactive. (Top) 293T cells were transfected with HA-tagged CP110 and Flag-tagged full-length or truncated fragments of USP33, extracts were immunoprecipitated, and western blots of the resulting immunoprecipitates were probed with anti-Flag and CP110 antibodies. WCE, whole cell extract.