Opinion statement
Although clear algorithms for diagnosis and treatment of patients with chest pain at low or high risk for an acute coronary syndrome (ACS) exist, they are less well delineated for patients presenting with chest pain with an intermediate risk for ACS. In patients presenting acutely or subacutely to emergency departments (EDs) at high risk for ACS, such as those with ST segment elevation on their 12-lead electrocardiogram (ECG), immediate contrast coronary angiography is performed. On the other hand, chest pain observation units (OUs) are recommended for managing those with chest pain at low risk for an ACS event. In this setting, these OUs are associated with lower healthcare resource utilization and improved cost-effectiveness. Cost-effective diagnosis and treatment options are important goals in healthcare delivery systems. The presentation of patients at intermediate risk for ACS represents an emerging source of resource utilization for EDs. These patients often exhibit pre-existing coronary artery disease, may have sustained prior myocardial infarction, and exhibit multiple comorbidities such as diabetes and hypercholesterolemia. Importantly, however, they will not have evidence of ST elevation on their 12-lead ECG nor will they exhibit serum markers (troponin or creatinine kinase elevations) indicative of ACS. As a consequence of existing co-morbidities, their management becomes time-consuming and may require inpatient monitoring, observation, and cardiac stress testing. Cardiovascular magnetic resonance (CMR) is a powerful tool for risk stratification and prognosis determination in patients in need of stress testing at intermediate risk of ACS. For those who present with acute chest pain syndromes, the combination of CMR in an OU setting represents a potentially attractive option for reducing healthcare-related expenditures without compromising patient outcomes. Recent study results from single centers suggest that CMR-OU care may result in fewer unnecessary hospital admissions and invasive procedures in those presenting with intermediate risk ACS. Further research utilizing stress CMR testing from multiple centers in OU settings is needed to determine if this model of care improves efficiency, reduces healthcare costs, and delivers optimum care in individuals presenting to EDs with chest pain at intermediate risk of ACS.
Keywords: Cardiac magnetic resonance, Observation units, Cost reduction, Stress testing
Introduction
Acute chest pain syndromes are a common reason for presentation to the emergency department (ED) [1]. Patients who present with an acute chest pain syndrome to the ED are initially triaged with an assessment of vital signs and an electrocardiogram (ECG), and then are further risk stratified based on the clinical presentation [2]. Cardiac biomarkers, often including troponin levels, are obtained on arrival. Diagnostic and therapeutic strategies for patients with symptoms consistent with acute coronary syndrome (ACS) are delineated in the American College of Cardiology/American Heart Association (ACC/AHA) guidelines [3]. The treatment strategies are clearer for patients with diagnostic ECG changes or elevated cardiac troponin results. These patients are usually admitted to the hospital, often in a coronary care unit, and receive aggressive antiplatelet, antithrombotic, and revascularization therapy. Patients without diagnostic ECG or biomarker changes undergo further testing with serial cardiac markers followed by cardiac stress testing, often employing cardiovascular (CV) imaging from an observation unit (OU) based on ACC/AHA ACS guidelines [3]. However, these patients without diagnostic ECG or biomarker results are heterogeneous with respect to risk for ACS and complicating features such as pre-existing heart disease. Often, these patients are further stratified into risk tiers, with lower-risk patients without prior cardiac events being managed commonly in chest pain OUs. High-risk patients are typically admitted to the hospital and receive more aggressive treatment and diagnostic testing with invasive cardiac catheterization and coronary angiography. Similar to high-risk patients, patients at intermediate risk are usually admitted to the hospital while undergoing evaluation with noninvasive cardiac imaging procedures and subsequently incur greater hospital costs and resource utilization.
Appropriate strategies for evaluation and treatment of patients at intermediate risk are less well defined than for low-risk or high-risk patients. In a randomized, controlled clinical trial of intermediate-risk patients who presented to the ED with unstable angina, investigators compared patients admitted for routine hospital admission to patients admitted to a chest pain OU where the median length of stay was 9.2 h [4]. They observed no significant difference in the rate of cardiac events, including nonfatal myocardial infarction (MI), death, acute congestive heart failure, stroke, or out-of-hospital cardiac arrest between the two groups during the first 6 months after discharge. Additionally, there was a 61% reduction in costs related to cardiac care over that same time period for those randomized to the OU-based strategy.
Several imaging modalities are used to risk stratify intermediate-risk patients. These include dobutamine stress echo (DSE) [5], single photon emission computed tomography (SPECT) [6], positron emission tomography (PET) [7], coronary computed tomography angiography (CTA) [8], or cardiovascular magnetic resonance (CMR) (Table 1). CMR-derived perfusion imaging and wall motion analysis, along with highly reproducible measurements of ventricular dimensions, function, and the ability to detect myocardial scar via late gadolinium enhancement, make CMR an excellent choice for risk stratification in patients who have an indication for stress testing. Strategies employing OU evaluation generally reduce length of stay and patients are often discharged from the unit within 24 h of presentation. This strategy incurs less healthcare-associated costs and reduces utilization of healthcare resources. The shorter length of hospitalization and lower costs also contribute to increased patient satisfaction [9]. Although not widely implemented, CMR-based OU strategies may combine the technical advantages of CMR stress testing with the focused, expedient care delivered by OUs to effectively manage patients with suspected ACS. In this review, we focus on the technical and financial implications of this strategy as indicated from the results of early, single-center, randomized controlled trials.
Table 1.
Advantages and disadvantages of noninvasive imaging modalities for diagnosing myocardial ischemia
| Imaging modality | Advantages | Disadvantages |
|---|---|---|
| Stress echocardiography | Provides functional data | Dependent on acoustic windows |
| Portable | Dependent on the sonographer | |
| High specificity | More subjective interpretation of wall motion abnormalities | |
| High inter-operator variability | ||
| SPECT | Provides functional data | Exposure to ionizing radiation |
| Well validated to assess prognosis | Worldwide shortage of technetium | |
| Available at most hospitals | Cost | |
| No contrast required | Longer scanning times | |
| PET | Provides functional data | Exposure to ionizing radiation and radionuclides |
| Superior attenuation correction and spatial resolution compared to SPECT | Cost | |
| Can be combined with CT | ||
| CT coronary angiography | Provides excellent anatomical data | Does not assess functional significance of lesions |
| Rapid | Exposure to ionizing radiation | |
| Requires iodinated contrast | ||
| Cardiac magnetic resonance | Provides excellent functional and anatomical data | Difficulty with ferromagnetic implants |
| No ionizing radiation or radionuclide exposure | Generally only available at larger institutions | |
| Not dependent on acoustic windows/can image in essentially any tissue orientation | Often utilizes gadolinium contrast |
PET positron emission tomography; SPECT single photon emission computed tomography
Vasodilator stress perfusion CMR
CMR imaging is a powerful tool for identifying patients at risk for adverse CV outcomes and is often used for diagnosing and evaluating myocardial ischemia and viability [10, 11]. CMR is being utilized more frequently due to its high temporal and spatial resolution, lack of exposure to ionizing radiation or radioisotopes, lack of reliance on acoustic windows [12], and its comprehensiveness for identifying both structural and physiologic myocardial and coronary abnormalities. Recent publications have demonstrated the utility of vasodilator stress CMR in patients presenting to EDs with acute chest pain syndromes [13].
The majority of cardiac imaging centers worldwide use vasodilators such as adenosine, regadenoson, or dipyridamole as the stress agents of choice during CMR. These agents decrease arteriolar resistance and increase coronary blood flow [14]. Coronary resistance in a region supplied by a flow-limiting stenosis is already reduced at baseline and myocardial blood flow increases are minimal or reduced after administration of a vasodilator; this is in contrast to other territories where blood flow is not impeded by stenotic coronary arteries and therefore increases markedly, creating a relative redistribution of blood flow to these myocardial segments and resulting in heterogeneous cardiac tissue blood flow.
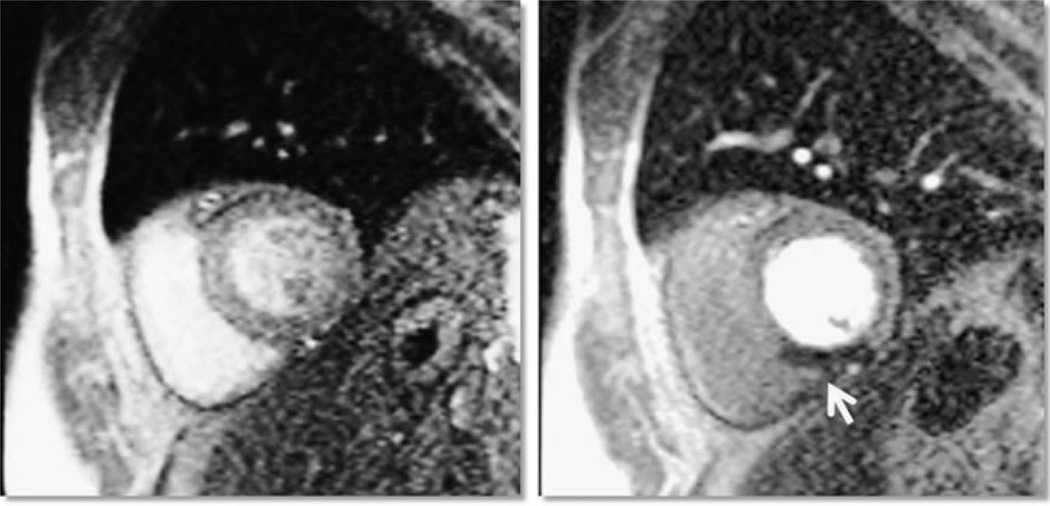
CMR is useful for detecting these areas of blood flow mismatch or perfusion defects. First pass T1-weighted images are obtained after gadolinium contrast is administered and then repeat imaging is performed (Fig. 1). Areas of myocardium that exhibit reduced contrast uptake that were not present at rest represent ischemia. Repeat imaging, usually performed 10 min later, can be utilized to detect myocardial necrosis, fibrosis, or scar using a technique known as late or delayed gadolinium enhancement (LGE or DE). Adenosine stress CMR has excellent negative predictive value for significant coronary artery disease (CAD) [15]. In a study of 158 predominantly intermediate-risk to high-risk patients referred for coronary angiography, Pilz et al. [16] performed adenosine stress perfusion and LGE CMR and observed a 96.2% negative predictive value of a normal adenosine stress CMR for significant CAD (defined as ≥70% stenoses).
Figure 1.
Bright blood parasternal short axis views of rest (left) and stress (right) perfusion images of a 62-year-old patient presenting to the emergency department with chest pain and a negative cardiac troponin. An inducible inferior defect (arrow) consistent with reversible ischemia was observed with adenosine stress. Subsequent invasive coronary angiography revealed a 95% right coronary artery stenosis.
Controlling cardiovascular image-related costs
Diagnosis of myocardial ischemia and functionally important CAD in patients at intermediate risk for ACS with abnormal ECGs is enhanced with the addition of noninvasive cardiovascular (CV) imaging procedures. CV imaging research is crucial to identifying optimum imaging pathways for patients with possible ACS. Although diagnostic imaging tests such as CMR may have high technical efficacy (ie, high accuracy) and prognostic significance in controlled settings, these attributes may not necessarily translate to societal gains [17]. Furthermore, research of these imaging modalities when applied to the population of interest, rather than the population in which they have been previously studied, identifies unique safety and technical issues associated with the application of these technologies. In response to rising healthcare costs, studies designed to look at a test’s cost-effectiveness are becoming increasingly important. Shortcomings in these key CV imaging research areas lead to ambiguity in defining the optimal imaging technique for each patient encounter. The net result is heterogeneous care patterns using a wide variety of CV imaging techniques. If these patterns lead to testing modalities being used in patients poorly suited for a particular technique, the result could be significant reductions in imaging quality and increases in imaging-associated healthcare expenditures.
Managing noninvasive cardiac imaging procedure use with radiology benefit managers
As expenditures for cardiac imaging have surpassed those of other physician services over the past several years [18], government and private insurance agencies have instituted policies designed to reduce imaging procedure use. Radiology benefit managers (RBMs) have been created to pre-certify cardiac imaging procedures including cardiac computed tomography (CCT) and CMR. Many have argued that imaging physicians are “self-referring” and that these physicians derive significant financial incentives from these procedures. RBMs have utilized provider exclusion from the imaging network, prior notification, and pre-certification as tools to blunt the dramatic rise in imaging-related expenditures [19].
Although there may be some financial benefits related to RBMs, mainly secondary to reductions in unnecessary testing, there are also several potential drawbacks, particularly if applied to patients suspected of ACS presenting to ED settings. Pre-certification is often utilized by RBMs and requires a formal application process that often has to be performed by the referring physician, often a primary care physician who has many other tasks to accomplish. This can be a time-consuming process and may deter some physicians from obtaining the most appropriate diagnostic tests for patients. Besides reduced timeliness of procedural completion due to these strategies and a generalized inconsistency and inefficiency associated with these programs, there is a dearth of evidence that utilization of RBMs actually improves quality of care. Additionally, RBMs may not be as applicable in acute care settings such as the ED, where cost savings and patient safety benefits might be jeopardized by time spent trying to meet criteria for pre-certification.
Quality and research to guide cost-effective ACS management
Physicians have advocated the use of quality measures to ensure that the correct imaging procedure is selected for appropriate patients [20]. In addition to ensuring that the imaging procedure is appropriate for the referred patient, imaging physicians are responsible for the adequate performance of the procedure as well as timely reporting of the results and summarizing the results in context of the patients’ clinical situations so that referring physicians can understand the results and incorporate them into better patient management. Quality control measures rely on evidence-based information to design clinical criteria, guidelines, and standards of care.
To this end, expert consensus documents and criteria for imaging modalities such as CMR and CCT have been published by respected physician societies such as the ACC to better guide physicians’ utilization of imaging procedures, including those designed to detect myocardial ischemia or injury [21•]. These documents are designed not only to educate physicians about the responsibility of ordering and providing high-quality testing procedures, but also serve to unify practice patterns of ordering physicians based on expert opinions and available evidence-based research.
In addition to Appropriateness Criteria, evidence-based outcomes research is used to define quality in imaging. Outcomes research can focus on the prognostic significance and cost-effectiveness of a test; both of which ultimately determine a test’s clinical utility. Recently, the results of several single center studies suggest beneficial clinical outcomes can be achieved while reducing healthcare costs [22•, 23•].
Lower downstream utilization cost with adenosine stress CMR
Like other imaging modalities, CMR is associated with an immediate increase in healthcare cost, but early data suggest that CMR may decrease downstream utilization and thus reduce cost in the intermediate term. In a German study by Pilz et al. [24] published in 2011, data from 218 patients from a CMR registry were matched from a previously identified low-risk group of patients based on CMR findings and then the proportion of patients in whom cardiac catheterization was deferred was estimated. They observed a 62% reduction in utilization of cardiac catheterization and a mean cost savings of € 90 (approximately $127), and this cost benefit was greatest in patients at lower risk for CAD.
In order to determine whether imaging with CMR in an OU would reduce costs in patients with emergent non–low-risk chest pain as opposed to a standard inpatient care strategy, Miller et al. [22•] conducted a randomized clinical trial among 110 ED participants at intermediate to high probability for ACS. These patients all had nondiagnostic ECGs and negative cardiac biomarkers and were randomized to adenosine stress CMR from the ED versus standard inpatient care. At 30 days after discharge, no subjects in either group experienced an ACS. The CMR group had a reduced median hospitalization cost of $2062 compared to standard inpatient care of $2680 for an estimated median difference of $588 (95% CI, $336–$881). The OU-CMR strategy was able to manage 79% of patients without inpatient admission and did not miss any patients with ACS. An example image from one of the study participants is shown in Fig. 1. This study evaluated patients who are normally admitted to an inpatient setting and often undergo more aggressive diagnostic testing. The two groups appeared to have similar costs related to ED costs, catheterization and revascularization cost, pharmacy cost, and provider cost. However, the cost savings from the OU-CMR strategy apparently were derived from reductions in laboratory testing and inpatient facility costs. In the inpatient care group there was a slightly higher number of patients who met criteria for ACS than in the observation group (6 versus 2). Although similar numbers of patients from each group underwent cardiac catheterization, fewer patients in the OU-CMR group underwent revascularization. This difference may be explained by interventionalists’ knowledge of the CMR results prior to catheterization compared to the catheterization being the first diagnostic imaging test.
Although Miller et al. [22•] showed a significant reduction in index hospitalization costs associated with an OU-CMR strategy in an intermediate-risk patient population, the costs associated with subsequent medical care, resource utilization, and clinical outcomes of these patients at later time points were unknown. Therefore, these participants were followed over 1 year from randomization for cardiac-related healthcare costs and utilization defined as cardiac-related hospital admissions, cardiac procedures, and cardiac-related office visits [23•]. They also analyzed secondary outcomes including MI, revascularization, and cardiovascular death. After discharge from the index hospitalization, significantly fewer patients in the OU-CMR strategy experienced cardiac-related ED visits (17% vs 37%, P=0.02) versus the inpatient care strategy. OU-CMR participants also observed a significantly lower number of subsequent cardiac-related hospital admissions (12% vs 35%, P=0.01). There were no statistically significant differences in the numbers of patients who underwent cardiac catheterization during the index hospitalization, but there was a significantly higher rate of subsequent catheterizations in the inpatient care group compared to the OU-CMR group after discharge (19% vs 2%, respectively, P=0.01). The mean cardiac-related costs over the 1-year follow-up period for the OU-CMR group were $3101 (95% CI, $2519–$3817) versus $4742 (95% CI, $3888–$5783) in the inpatient care group over the same time period (P=0.004). At 1 year, there was no significant difference in major cardiac events in the two groups (6% in the OU-CMR group vs 9% in the inpatient group). These results demonstrate that the utilization reductions observed in the index visit were not associated with a subsequent increase in utilization after discharge as might be expected if care were deferred to the outpatient setting. In fact, the OU-CMR group continued to experience reductions in healthcare utilization after discharge suggesting that this care strategy impacts downstream behaviors.
Based on current ACC/AHA Guidelines [3], admission to chest pain OUs is a Class I recommendation for patients without definite ACS. Based on data from the single-center clinical trials discussed above, it may be reasonable to monitor non–low-risk or intermediate-risk patients with nondiagnostic ECGs and negative cardiac biomarkers in OUs with stress CMR protocols. Although Ramakrishna et al. [25] did observe cost savings when intermediate-risk patients were placed in an OU, they did not observe a continued cost savings at 6 months when imaging was added to stress testing. It is unclear whether the cost savings accumulated during the 1-year follow-up study by Miller et al. [23•] were attributable to the utilization of CMR or a combination of the OU-CMR strategy. The addition of CMR did lower rates of cardiac catheterizations in the follow-up period, which contributed to reduced costs in this group. The lower percentage of patients presenting to the ED for cardiac-related conditions could be a result of patients’ satisfaction and reassurance with their diagnoses based on an advanced imaging test.
Technical and safety issues
CMR stress imaging is a safe test when appropriately administered and has several advantages when compared to other imaging modalities, including the lack of ionizing radiation or isotope exposure as well as the lack of a need for iodinated contrast administration. The same general safety concerns that apply to any other form of MR imaging generally apply to CMR as well, including having a specialized room protected from ferromagnetic objects. Patients are screened by knowledgeable MR technologists or physicians for cardiac devices or other implants. Most devices are classified as either “MR safe, conditional, or unsafe” by the American Society of Testing and Materials [26].
Gadolinium contrast is typically administered for stress perfusion CMR studies and is associated with a low rate of adverse events. However, a rare condition termed nephrogenic systemic fibrosis/sclerosis, a fibrosing scleroderma-like condition affecting multiple organ systems, has been identified as a complication in patients with acute kidney injury or chronic renal insufficiency (defined as glomerular filtration rate <30 mL/min/1.73 m2) [27].
The adverse event rates associated with the administration of inotropic or vasodilator stress agents during CMR stress exams are comparable to or lower than those reported with other imaging modalities [28, 29]. Overall, stress CMR is safe as evidenced by data from a multicenter registry of over 11,000 patients (88% received gadolinium-based contrast) who underwent CMR in which only 0.05% of patients developed severe complications, and no deaths occurred at the time of CMR exam or due to CMR [30]. Criteria for performing as well as reporting stress CMR studies have been established by the Society for Cardiovascular Magnetic Resonance (http://www.scmr.org/navigation/CMR-in-specific-circumstances/1462.html).
Summary
Acute chest pain syndromes are a common reason for presentation to the ED and result in significant health-care-associated expenditures. As a result of exponential growth with CV imaging utilization, government and private sectors have implemented policies designed to restrain costs associated with the performance of these procedural tests. Two recent analyses by Miller et al. [22•, 23•] demonstrated significant short-term and long-term cost savings in patients with non–low-risk chest pain with an OU-CMR strategy as opposed to standard inpatient care. Adenosine stress perfusion CMR has excellent negative predictive value in both low-risk and non–low-risk patients being evaluated for CAD and appears to be a cost-effective diagnostic modality with an excellent safety profile. Further larger, multicenter studies are necessary to better define the role of adenosine stress CMR in the setting of non–low-risk chest pain and to help guide practice patterns of physicians in relation to ordering and performing CV imaging examinations for improved and cost-effective patient management.
Acknowledgments
Funded in part by the Translational Science Institute of Wake Forest University School of Medicine; NIH Grants 1 R21 HL097131-01A1 (Miller), 1 RO1 HL076438 (Hundley), and 1 R33 CA12196 (Hundley) and American Heart Association Grant 0980008N (Miller)
Footnotes
Disclosure
M.E. Hall: none; C.D. Miller has received research grants from EKR therapeutics (significant), Johnson & Johnson/Scios Inc., Chiron Corporation, and Astra Pharmaceuticals; has received research support from Siemens; and has been an expert witness for Up-to-Date; W.G. Hundley has received research support from Bracco Diagnostics and owns stock/stock options in Prova, Inc.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Rosamond W, Fiegal K, Friday G, et al. Heart disease and stroke statistics-2007 an update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e71. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Selker HP, Zaleski RJ, Antman EM, et al. An evaluation of technologies for identifying acute cardiac ischemia in the emergency department: a report from a National Heart Attack Alert Program Working Group. Ann Emerg Med. 1997;29:1–12. doi: 10.1016/s0196-0644(97)70297-x. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 Guidelines for the Management of Patients with Unstable Angina/Non ST-Elevation Myocardial Infarction. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients with Unstable Angina/Non ST-Elevation Myocardial Infarction) Circulation. 2002;2007(116):e148–e304. doi: 10.1161/CIRCULATIONAHA.107.181940. [DOI] [PubMed] [Google Scholar]
- 4.Farkouh ME, Smars PA, Reeder GS, et al. A clinical trial of a chest-pain observation unit for patients with unstable angina. Chest Pain Evaluation in the Emergency Room (CHEER) Investigators. N. Engl J. Med. 1998;339:1882–1888. doi: 10.1056/NEJM199812243392603. [DOI] [PubMed] [Google Scholar]
- 5.Afridi I, Quinones MA, Zoghbi WA, et al. Dobutamine stress echocardiography: sensitivity, specificity, and predictive value for future cardiac events. Am Heart J. 1944;127:1510–1515. doi: 10.1016/0002-8703(94)90378-6. [DOI] [PubMed] [Google Scholar]
- 6.Hilton TC, Fulmer H, Abuan T, et al. Ninety-day follow-up of patients in the emergency department with chest pain who undergo initial single-photon emission compute tomographic perfusion scintigraphy with technitium 99m-labeled sestamibi. J Nucl Cardiol. 1996;3:308–311. doi: 10.1016/s1071-3581(96)90090-2. [DOI] [PubMed] [Google Scholar]
- 7.Mammen L, White RD, Woodard PK, et al. ACR appropriateness criteria on chest pain, suggestive of acute coronary syndrome. J Am Coll Radiol. 2011;8:12–18. doi: 10.1016/j.jacr.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 8.Maffei E, Seitun S, Martini C, et al. CT coronary angiography and exercise ECG in a population with chest pain and low-to-intermediate pre-test likelihood of coronary artery disease. Heart. 2010;96:1973–1979. doi: 10.1136/hrt.2009.191361. [DOI] [PubMed] [Google Scholar]
- 9.Rydman RJ, Zalenski RJ, Roberts RR, et al. Patient satisfaction with an emergency department chest pain observation unit. Ann Emerg Med. 1997;29:109–115. doi: 10.1016/s0196-0644(97)70316-0. [DOI] [PubMed] [Google Scholar]
- 10.Hundley WG, Morgan TM, Neagle CM, et al. Magnetic resonance imaging determination of cardiac prognosis. Circulation. 2002;106:2328–2333. doi: 10.1161/01.cir.0000036017.46437.02. [DOI] [PubMed] [Google Scholar]
- 11.Jahnke C, Nagel E, Gebker R, et al. Prognostic value of cardiac magnetic resonance stress tests: adenosine stress perfusion and dobutamine stress wall motion imaging. Circulation. 2007;115:1769–1776. doi: 10.1161/CIRCULATIONAHA.106.652016. [DOI] [PubMed] [Google Scholar]
- 12.Hundley WG, Hamilton CA, Thomas MS, et al. Utility of fast cine magnetic resonance imaging and display for the detection of myocardial ischemia in patients not well suited for second harmonic stress echocardiography. Circulation. 1999;100:1697–1702. doi: 10.1161/01.cir.100.16.1697. [DOI] [PubMed] [Google Scholar]
- 13.Kwong RY, Schussheim AE, Rekhraj S, et al. Detecting acute coronary syndrome in the emergency department with cardiac magnetic resonance imaging. Circulation. 2003;107:531–537. doi: 10.1161/01.cir.0000047527.11221.29. [DOI] [PubMed] [Google Scholar]
- 14.Udelson J, Dilsizian V, Bonow RO. Nuclear cardiology. In: Libby P, Bonow RO, Mann DL, Zipes DS, editors. Braunwald’s heart disease: a textbook of cardiovascular medicine. Philadelphia: Saunders; 2008. pp. 345–391. [Google Scholar]
- 15.Lerakis S, McLean DS, Anadiotis AV, et al. Prognostic value of adenosine stress cardiovascular magnetic resonance in patients with low-risk chest pain. J Cardiovasc Magn Reson. 2009;11:37. doi: 10.1186/1532-429X-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pilz G, Eierle S, Heer T, et al. Negative predictive value of normal adenosine-stress cardiac MRI in the assessment of coronary artery disease and correlation with semiquantitative perfusion analysis. J Magn Reson Imaging. 2010;32:615–621. doi: 10.1002/jmri.22289. [DOI] [PubMed] [Google Scholar]
- 17.Fryback DG, Thornbury JR. The efficacy of diagnostic imaging. Med Decis Making. 1991;11:88–94. doi: 10.1177/0272989X9101100203. [DOI] [PubMed] [Google Scholar]
- 18.Koening L, Dobson A, Book R, et al. The Lewin Group. Prepared for the American College of Cardiology. Falls Church, VA: The Lewin Group; 2005. Issues in the growth of diagnostic imaging services. A case study of cardiac imaging. [Google Scholar]
- 19.Hendel RC. Utilization management of cardiovascular imaging: pre-certification and appropriateness. J Am Coll Cardiol Img. 2008;1:241–248. doi: 10.1016/j.jcmg.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Douglas PS, Chen J, Gilliam L, et al. Achieving quality in cardiovascular imaging II: proceedings from the second American College of Cardiology-Duke University Medical Center think tank on quality in cardiovascular imaging. J Am Coll Cardiol Img. 2009;2:231–240. doi: 10.1016/j.jcmg.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 21. Hundley WG, Bluemke DA, Finn JP, et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Task Force on Expert Consensus Documents. J Am Coll Cardiol. 2010;55:2614–2662. doi: 10.1016/j.jacc.2009.11.011. These recently published expert consensus recommendations give an excellent up-to-date overview on the current functional and anatomical uses for cardiac MRI as well as appropriateness and safety considerations for this technology.
- 22. Miller CD, Wenke H, Hoekstra JW, et al. Stress cardiac magnetic resonance imaging with observation unit care reduces cost for patients with emergent chest pain: a randomized trial. Ann Emerg Med. 2010;56:209–219. doi: 10.1016/j.annemergmed.2010.04.009. This randomized trial demonstrated reduced incident costs without any missed cases of ACS in intermediate-risk patients with emergent chest pain who weremanaged in an OU setting with adenosine stress CMR.
- 23. Miller CD, Hwang W, Case D, et al. In emergency department patients with acute chest pain, stress cardiac MRI observation unit care reduces 1-year cardiac-related health care expenditures: a randomized trial. J Am Coll Cardiol Img. (in press). This follow-up study demonstrated significant short-term and long-term cost savings in patients with non–low-risk chest pain with an OU-CMR strategy as opposed to standard inpatient care.
- 24.Pilz G, Patel PA, Fell U, et al. Adenosine-stress cardiac magnetic resonance imaging in suspected coronary artery disease: a net cost analysis and reimbursement implications. Int J Cardiovasc Imaging. 2011;27:113–121. doi: 10.1007/s10554-010-9645-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramakrishna G, Milavetz JJ, Zinsmeister AR, et al. Effect of exercise treadmill testing and stress imaging on the triage of patients with chest pain: CHEER substudy. Mayo Clin Proc. 2005;80:322–329. doi: 10.4065/80.3.322. [DOI] [PubMed] [Google Scholar]
- 26.Shellock FG, Spinazzi A. MRI safety update 2008: Part 2, screening patients for MRI. AJR Am J Roentgenol. 2008;191:1–10. doi: 10.2214/AJR.08.1038.2. [DOI] [PubMed] [Google Scholar]
- 27.Kuo PH, Kanal E, Abu-Alfa AK, et al. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology. 2007;242:647–649. doi: 10.1148/radiol.2423061640. [DOI] [PubMed] [Google Scholar]
- 28.Kuijpers D, Jannsen CH, van Dijkman PR, et al. Dobutamine stress MRI: Part 1. Safety and feasibility of dobutamine cardiovascular magnetic resonance imaging in patients suspected of myocardial ischemia. Eur Radiol. 2004;14:1823–1828. doi: 10.1007/s00330-004-2425-y. [DOI] [PubMed] [Google Scholar]
- 29.Karamitsos TD, Arnold JR, Pegg TJ, et al. Tolerance and safety of adenosine stress perfusion cardiovascular magnetic resonance imaging in patients with severe coronary artery disease. Int J Cardiovasc Imaging. 2009;25:277–283. doi: 10.1007/s10554-008-9392-3. [DOI] [PubMed] [Google Scholar]
- 30.Bruder O, Schneider S, Nothnagel D, et al. EuroCMR (European Cardiovascular Magnetic Resonance) registry: results of the German pilot phase. J Am Coll Cardiol. 2009;54:1457–1466. doi: 10.1016/j.jacc.2009.07.003. [DOI] [PubMed] [Google Scholar]