Abstract
Purpose
To assess if the impact of partial nephrectomy (PN) and radical nephrectomy (RN) on overall mortality differed by patient age in a Medicare population undergoing surgery for T1a Renal Cell Carcinoma (RCC).
Materials and Methods
Using linked Surveillance, Epidemiology and End Results -Medicare data, we identified patients >66 years of age who underwent PN or RN for T1a (≤4cm) RCC from 1995–2007. The effects of procedure type on overall mortality by age were assessed using time dependent Cox proportional hazards models adjusted by propensity score based weighting.
Results
5,496 patients (mean age 74.2±5.6 years, 55.9% male) who underwent PN (n=1,665; 30.3%) or RN (n=3,831; 69.7%) for ≤4cm RCC (mean tumor size 2.8±0.9cm) were identified. Following adjustment, a statistically significant survival benefit for PN compared to RN was observed at one (age 68: HR 1.6 [CI 1.2–2.3]; age 75: HR 1.5 [CI 1.1–1.9]; age 85: HR 1.7 [CI 1.1–2.5]) and three (age 68: HR 1.4 [CI 1.03–2.0]; age 75: HR 1.3 [CI 1.1–1.6]; age 85: HR 1.5 [CI 1.02–2.3]) years while these trends become insignificant in patients <68 and >85 years of age. However, the survival benefit diminished over time, and little significant benefit with PN was observed at 5 and 10 years following surgery regardless of age (≥66 years).
Conclusions
Lacking strong evidence regarding a long term survival benefit, the decision to perform PN in elderly patients should be individualized and placed in the context of baseline renal function, expected surgical morbidity, and competing risks to survival.
Keywords: kidney, carcinoma, nephrectomy, partial nephrectomy, survival, nephron preservation, competing risks
Introduction
In 2012 it is estimated that more than 64,770 patients will be diagnosed with kidney cancer, predominantly renal cell carcinoma (RCC), and 13,570 will die from their disease.1 Increased use of abdominal imaging has resulted in a dramatic stage migration towards smaller, localized renal masses, and as a result the incidental detection of asymptomatic stage I (≤7cm) lesions now accounts for more than half of new diagnoses.2 While alternative management strategies such as ablation and active surveillance are currently under investigation3, the gold standard remains surgical excision. Traditionally managed with radical nephrectomy (RN), concerns that significant loss of renal parenchyma may predispose patients to the sequelae of chronic kidney disease (CKD) including increased cardiovascular risk and shortened overall survival (OS)4, 5, have resulted in experts recommending nephron sparing surgery (NSS) for all amenable clinical stage I lesions.6
To date, evidence suggesting a survival benefit with NSS has been limited to retrospective institutional or secondary data analyses7–9, which suffer from selection biases and lack of generalizability. In 2011, the European Organization for the Research and Treatment of Cancer (EORTC) reported results from a phase III trial randomizing 541 patients with solitary renal lesions ≤5cm and a normal contralateral kidney to partial nephrectomy (PN) or RN.10 While oncologic efficacy was largely equivalent between treatment arms, the authors reported an unanticipated 10 year OS benefit favoring patients treated with RN. While limited due to poor accrual and substantial cross over between treatment groups, these data do raise provocative questions regarding the impact of NSS on survival in elderly patients.11 Although previous reports have advocated performance of PN in all patients when technically feasible7, the decision to perform a PN in an elderly or infirmed patient requires careful consideration of competing risks to mortality12 given the increased risk of surgical morbidity following NSS.13 Since it is unlikely that additional prospective surgical trials for localized disease will be conducted, optimally adjusted observational studies may represent the best available method to determine which patients stand to benefit the most from NSS. Hypothesizing that the survival benefit with NSS become less pronounced in patients of advancing age, we assessed if the associations between PN, RN, and overall mortality differed by patient age and time from surgery in a Medicare population undergoing surgery for T1a RCC.
Material (Patients) and Methods
The Surveillance, Epidemiology and End Results (SEER) program, as reported by the US National Cancer Institute, collects patient demographics and publishes incidence and survival data from population-based cancer registries, currently covering approximately 26% of the US population. Among patients 65 years or older with a cancer diagnosis recorded in the SEER data, 94% have been linked with Medicare enrollment data.14
Study inclusion criteria included Medicare beneficiaries for whom kidney cancer (stage 1a [≤4cm, common histologies: clear cell, papillary, chromophobe, and adenocarcinoma]) was their first lifetime kidney cancer diagnosis. We only included patients who had Medicare part A and B coverage for one year prior and one year after cancer diagnosis (unless death occurred in less than one year). Demographic characteristics, cancer severity, cause of death, and procedure assignment were derived from the SEER PEDSF file. For each identified case, we searched both inpatient (Medicare Provider Analysis and Review [MEDPAR] file), outpatient (OUTSAF*), and physician claims (NCH) files to determine burden of comorbidity defined by the Charlson Comorbidity Index (CCI)15, 16 and pre-treatment chronic renal failure defined by the Elixhauser method17. We restricted the study sample to subjects who were ≥66 years at diagnosis to ensure that all subjects had ≥1 year of claims data from which to derive comorbidity.
Data Analyses
Unadjusted patient characteristics were compared between procedure groups using ANOVAs and chi-squared tests. To account for patient level differences between treatment groups, we used adjustment via propensity score-based weighting.18 A logistic regression model was used to determine the probability of undergoing PN or RN for each patient in the dataset. Covariates included in the propensity score model were sex, race, year of diagnosis, CCI, marital status, area of residence (urban/rural), tumor size, histologic subtype, tumor grade, year treated, SEER region, and three census tract measures of socioeconomic status. Age was included in the propensity score model for survival analyses in which an age interaction on the RN effect was not of primary interest. For each covariate, we assessed whether adjustment was adequate by examining logistic regressions of RN, adjusted by propensity-score based weights, with the covariate as an independent variable.
OS differences were assessed using unadjusted and adjusted Kaplan-Meier curves respectively.19 For our adjusted disease-specific mortality analysis, we used a Fine and Gray competing risk regression with propensity score (including age as a covariate) adjustment via weighting.20 The RN indicator was the sole covariate in the model. For OS analyses, we used Cox proportional hazard regressions adjusted by propensity score-based weights. For the Cox regression models we did not include age in the propensity score as we were investigating age as a moderator of treatment effect. Treatment was included in the weighted Cox models as both a baseline (non-time varying covariate) and a time-varying covariate. In addition we included age and the interaction of age and treatment as baseline and time varying covariates in a model designed to investigate whether age moderated the effect of treatment. Age at diagnosis was entered into the models via a restricted cubic spline with four knots at empirical quantiles (0.05, 0.35, 0.65, 0.95 quantiles). In order to investigate whether the effect of RN varied over time, we also included the RN covariate and the three spline term interactions into the model as time dependent covariates. We used the bootstrap method with 1000 random samples to obtain the standard errors in the propensity-score weighted regression analyses.21 All analyses were conducted using STATA version 10 (StataCorp, College Station, Texas), with p values of <0.05 meeting statistical significance.
Results
Between 1995 and 2007, 6,354 patients surgically treated for localized kidney cancers ≤4cm who were ≥66 years in the SEER registry with sufficient Medicare Part A and B coverage were identified. After excluding patients with missing covariate data, the final sample for analysis included 5,496 patients with a mean follow up of 4.4±2.8 years (median 4, range .02–13.8 years). Mean age, CCI, and proportion of patients with pre-treatment chronic renal failure were 74.2±5.6 years (median 74, range 66–95 years), 0.85±1.2 (median 0; range 0–10), and 4.4% respectively, in a cohort of patients who were predominantly male (56%), married (63%), and Caucasian (85%). Mean tumor size was 2.8±0.9cm (median 3 cm; range 0.1–4.0 cm), and of patients with available data the majority of lesions were of clear cell histologic type (83%) and low grade (82%).
Patients were classified by treatment type: RN (n=3,831; 69.7%) and PN (n=1,665; 30.3%). In the unadjusted analyses, significant differences between treatment groups were observed in patient age (p<0.001), gender (p<0.001), marital status (p=0.01), geographic area of residence (p=0.002), SEER region (p<0.001), median household income (p<0.001), percent of patients >25 years of age with <12 years of education (p=0.003), and length of follow up (p<0.001). Comparing clinical characteristics, significant differences were observed with respect to tumor size (p<0.001) and histologic type (<0.01), while no differences were demonstrated with respect to pre-treatment chronic renal failure, CCI, or tumor grade. Stratified by propensity weight for treatment received, no significant differences in demographic, clinical, or pathologic characteristics were observed between groups.
Using a competing risk regression with propensity score-based weights for adjustment and RN as a covariate, we found that the effect of RN on cancer specific survival was not statistically significant (HR 1.42, [CI 0.92–2.20], p=0.114). In contrast, we present unadjusted and adjusted analyses revealing significant differences in OS (all p<0.0001) when stratified by treatment type (Figure 1). Comparing time dependent differences in OS by age, a statistically significant survival benefit for PN compared to RN was observed at one (diagnosis age 68: HR 1.6 [CI 1.03–2.3]; age 75: HR 1.5 [CI 1.1–1.9]; age 85: HR 1.7 [CI 1.1–2.5]) and three (diagnosis age 68: HR 1.4 [CI 1.04–2.0]; age 75: HR 1.3 [CI 1.1–1.6]; age 85 1.5 [CI 1.02–2.3]) years while these trends become insignificant in patients <68 and >85 years of age (Figure 2). However, this benefit diminished over time, and little significant survival benefit with PN was observed at 5 and 10 years following surgery regardless of age (Figure 3).
Figure 1.
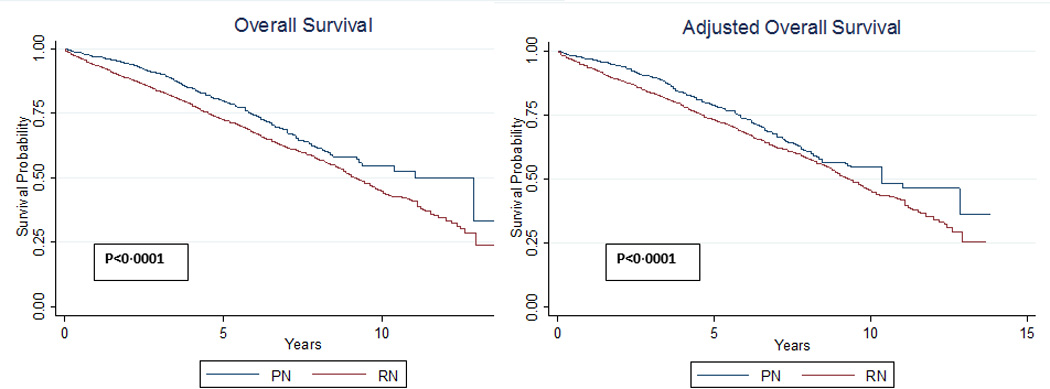
Unadjusted and adjusted overall mortality stratified by receipt of partial (PN) or radical nephrectomy (RN).
Figure 2.
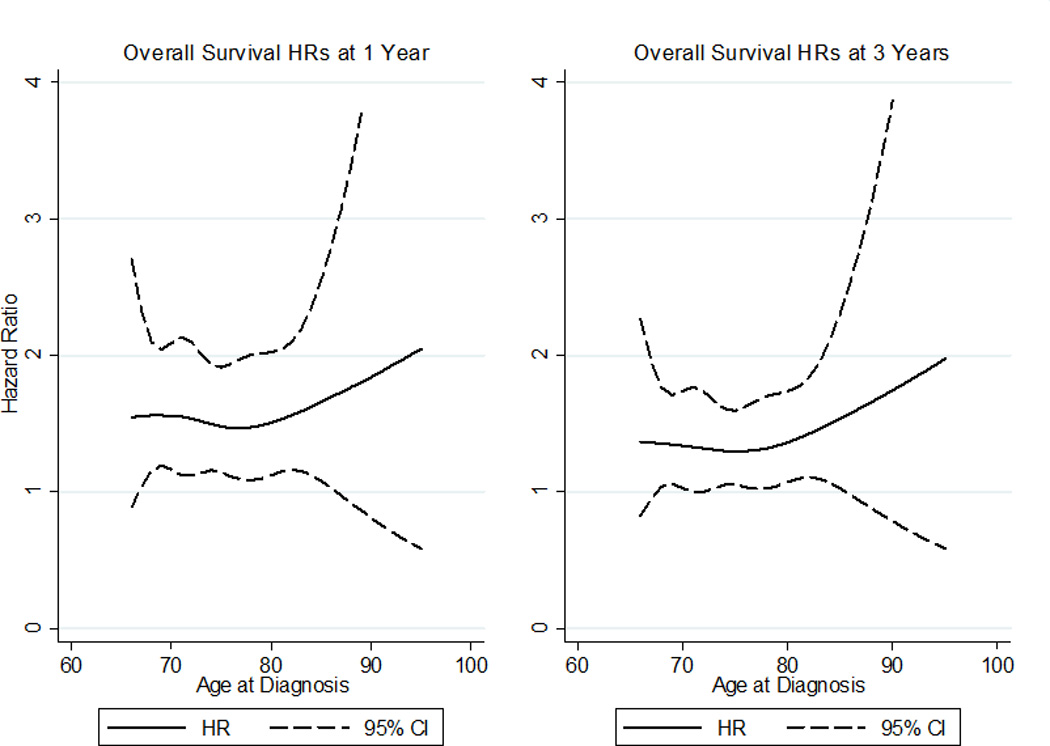
Cox hazard ratios (HRs) for overall survival by diagnosis age at 1 and 3 years post-diagnosis. HRs>1 indicate radical nephrectomy is harmful compared to partial nephrectomy
Figure 3.
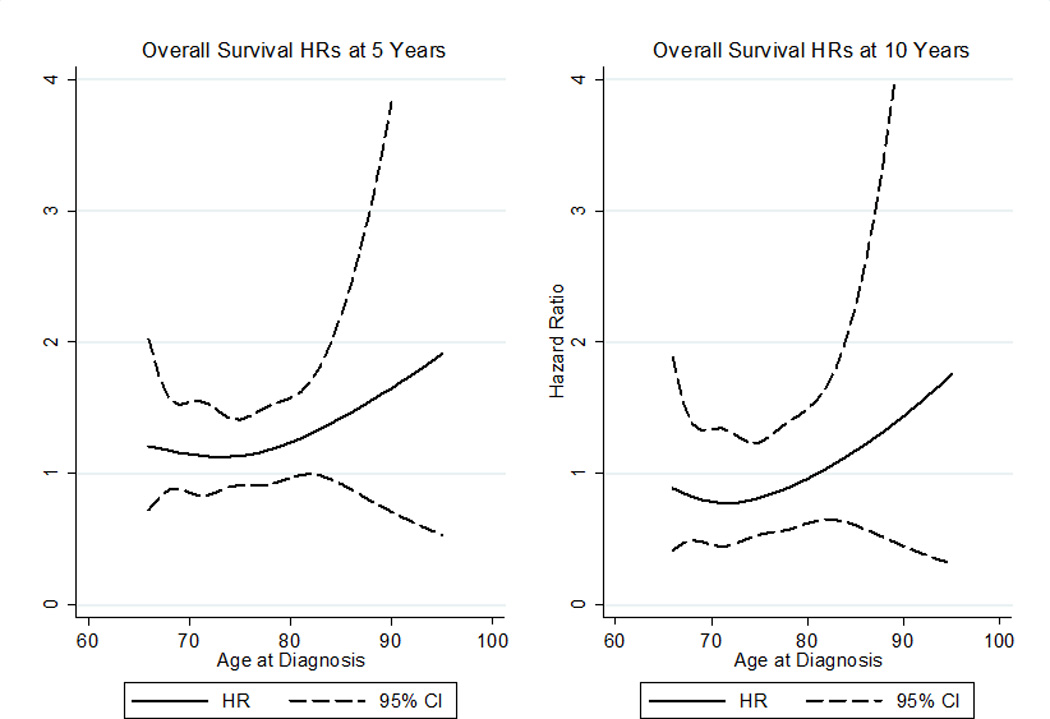
Cox hazard ratios (HRs) for overall survival by diagnosis age at 5 and 10 years post-diagnosis. HRs>1 indicate radical nephrectomy is harmful compared to partial nephrectomy
In a propensity score-based weight adjusted Cox model in which RN was entered as a covariate and as an interaction term with time the baseline effect of RN was strong (HR 1.7 [CI 1.3–2.1]). However, the effect of RN decreased over time (HR 0.9 [CI 0.9–0.99] for interaction of RN with time). This suggests that the harmful effect of RN versus PN diminishes over time, and is consistent with Figure 1 in which the distance between survival curves interpose with increasing time from diagnosis. Repeating analyses restricted to patients without documented chronic renal failure (N=5,255) or tumor grade (N=4,071) did not significantly impact our findings (data not presented).
Discussion
Over the past 20 years, a rise in the incidental detection of localized renal masses has been matched by an increase in the number of renal surgeries performed.2 Due to efforts to preserve functional renal parenchyma and reduce the incidence of cardiovascular events and death4, experts have advocated for the increased utilization of NSS, which has been incorporated into existing best practice guidelines as standard of care for stage I renal masses.6 While the rationale for PN is well established, NSS has been slow to gain traction in the urologic community and currently remains underutilized.22, 23
To date, institutional and administrative analyses have demonstrated a reduced risk of CKD and adverse renal functional outcomes following PN5, 24, while less definitive associations have been reported regarding the association between treatment received, cardiovascular outcomes, overall, and other cause mortality.7–9, 24, 25 Adequately adjusting for severity and selection bias in the markedly heterogenous population of patients undergoing cancer directed surgery represents the largest hurdle in contemporary observational studies.26 These challenges stem from the fact that utilization of PN differs among providers based on tumor, hospital, and patient characteristics23, 27, resulting in variation that is challenging to capture using administrative data. With measured tumor characteristics limited to size, stage, histology, and grade, administrative datasets are unable to adequately capture tumor complexity, and no study examining survival outcomes has adequately controlled for pre-operative renal function. While statistical methods such as propensity matching and adjustment reduce selection bias by controlling for observed confounding factors that are not biased by treatment outcome18, there are unmeasured factors that likely influence survival outcomes following kidney surgery. Further, recent transplant data fails to show a survival difference or increased risk of end-stage renal disease in patients undergoing donor nephrectomy compared to the general population.28 While patients undergoing donor nephrectomy have important differences from patients undergoing surgery for RCC, these data underscore that the physiologic implications of a normally functioning solitary kidney have yet to be fully established.11
In our study of Medicare beneficiaries, we controlled for selection bias between treatment groups using propensity score based weighting, an accepted method of covariate adjustment which is similar in principle to propensity matching but does not reduce the sample size of comparison groups.18 Similar to prior reports7–9, 25, PN in our cohort was associated a with a short term significant survival advantage at one and three years in patients 68 to 85 years of age. Furthermore, PN appeared to be advantageous in patients >85 years of age (HR >1) but was not statistically significant, perhaps due to the small sample size. However, if the mechanism for the survival advantage afforded by NSS is a result of improved metabolic and cardiovascular function, one would expect that these effects become more pronounced as the time interval from the date of surgery increases. In contrast, we could not demonstrate a survival advantage with NSS at 5 or 10 years from surgery in Medicare beneficiaries of any age, which implies that some patients are destined to expire within 5 years of surgery, while others have lower near term but persistent long term risks.29 Among this latter group, the treatment received may become less important as the time from diagnosis increases. Although time-varying treatment effects have been demonstrated in patients with breast cancer undergoing adjuvant therapy29, 30, to our knowledge this phenomenon has not been evaluated in patients treated for localized kidney cancer.
Our finding that the early protective effect of PN observed in the SEER-Medicare dataset was not durable over time can be interpreted in a number of ways. One could assume that the majority of patients in our cohort with severe competing risks or pathologic characteristics died within the first 5 years of treatment, leaving a more homogenous group of survivors in which the benefits of nephron preservation may be more subtle than anticipated. However, it is also feasible that the effect on OS was dampened due to the influence of unobserved differences not captured using Medicare claims, which may be amplified in a more generalizable patient cohort.
While these findings are provocative, our study is limited by lack of generalizability, unavailable anatomic and physiologic data, limitations of current comorbidity quantification methodology, and the inability to determine indication for NSS.11, 26 Nevertheless, Medicare data currently represents the most robust dataset for investigating clinical outcomes in elderly patients, which was our sample of interest. Our analysis sheds some light on the unanticipated survival findings of the EORTC trial10, and provides new insight that the benefit of NSS surgery in elderly patients may be less than expected and may not be durable over time. Consistent with prior reports8, we anticipate that younger patients, particularly with pre-existing risk factors for CKD, represent a group in which a persistent survival advantage may be more easily demonstrable, but future investigations will require more robust data sources including all payer groups as well as clinical/physiologic data that is not limited by age.
Conclusions
In conclusion, we demonstrate a time varying treatment benefit for partial versus radical nephrectomy in Medicare beneficiaries that does not appear durable over time. With presentation of these data, we must stress that we remain strong advocates for NSS. Prevention of CKD is critical4, NSS is underutilized22, 23, and PN, which in experienced hands is technically feasible in nearly all cases, should be performed in appropriate candidates.6 Nevertheless, the increased risk of morbidity with PN may offset a potential survival benefit in elderly or comorbid patients with a normal contralateral kidney. While our intention is not to discourage use of PN, our findings illustrate that further study is required to identify the select patients for whom the increased risks of NSS may outweigh the potential benefits. Further, improved understanding of the relationship between CKD and cardiovascular risk factors will contribute greatly to refinement of existing risk stratification tools and facilitate informed individualized decision making.12 Until better understanding of these relationships has been achieved, we must continue to individualize care for patients with localized RCC by attempting to objectify competing risks and using clinical judgment to guide critical decision-making.
Table I.
Unadjusted demographic and tumor characteristics of the study population
| Characteristic | All Patients | Partial Nephrectomy |
Radical Nephrectomy |
P Value |
|---|---|---|---|---|
| N | 5,496 | 1,665 | 3,831 | |
| mean±SD; N(%) | ||||
| Age (years) | 74.2±5.6 | 73.3±5.2 | 74.5±5.7 | <0.001 |
| Tumor size (cm) | 2.8±0.9 | 2.5±0.8 | 3.0±0.8 | <0.001 |
| Charlson Co-Morbidity Index | 0.85±1.2 | 0.86±1.2 | 0.85±1.3 | 0.853 |
| Pre-existing Chronic Renal Failure | 241 (4.4) | 65 (3.9) | 176 (4.6) | 0.282 |
| Gender | ||||
| Male | 3,072 (55.9) | 992 (59.6) | 2,080 (54.3) | <0.001 |
| Female | 2,424 (44.1) | 673 (40.4) | 1,751 (45.7) | |
| Race/Ethnicity | 0.249 | |||
| Caucasian | 4,694 (85.4) | 1,422 (85.4) | 3,272 (85.4) | |
| African American | 462 (8.4) | 141 (8.5) | 321 (8.4) | |
| Asian | 102 (1.9) | 37 (2.2) | 65 (1.7) | |
| Hispanic | 120 (2.2) | 27 (1.6) | 93 (2.4) | |
| Other | 118 (2.2) | 38 (2.3) | 80 (2.1) | |
| Marriage Status | 0.01 | |||
| Not married | 2,061 (37.5) | 579 (34.8) | 1,482 (38.7) | |
| Married | 3,435 (62.5) | 1,086 (65.2) | 2,349 (61.3) | |
| Area of residence | 0.002 | |||
| Non-metropolitan | 807 (14.7) | 207 (12.4) | 600 (15.7) | |
| Metropolitan | 4,689 (85.3) | 1,458 (87.6) | 3,231 (84.3) | |
| Histologic Type | <0.01* | |||
| Clear cell | 4,573 (83.2) | 1,285 (77.2) | 3,288 (85.8) | |
| Papillary | 615 (11.2) | 265 (15.9) | 350 (9.1) | |
| Chromophobe | <300 (<6%) | <120 (<7%) | <200 (<5%) | |
| Adenocarcinoma | <20 (<1%) | <11 (<1%) | 20 (<1%) | |
| Grade | 0.233 | |||
| 1 | 983 (17.9) | 325 (19.5) | 658 (17.2) | |
| 2 | 2,341 (42.6) | 702 (42.2) | 1,639 (42.8) | |
| 3 | 676 (12.3) | 201 (12.1) | 475 (12.4) | |
| 4 | 71 (1.3) | 17 (1.0) | 54 (1.4) | |
| missing | 1,425 (25.9) | 420 (25.2) | 1,005 (26.2) | |
| SEER Region | <0.001 | |||
| San Francisco | 167 (3.0) | 65 (3.9) | 102 (2.7) | |
| Connecticut | 413 (7.5) | 126 (7.6) | 287 (7.5) | |
| Detroit | 538 (9.8) | 142 (8.5) | 396 (10.3) | |
| Hawaii* | <80 (<2%) | <30 (<2%) | <60 (<2%) | |
| Iowa | 425 (6.3) | 105 (6.3) | 320 (8.4) | |
| New Mexico | 105 (1.9) | 21 (1.3) | 84 (2.2) | |
| Seattle | 310 (5.6) | 105 (6.3) | 205 (5.4) | |
| Utah | 113 (2.1) | 38 (2.3) | 75 (2.0) | |
| Atlanta | 122 (2.2) | 38 (2.3) | 84 (1.2) | |
| San Jose | 103 (1.9) | 23 (1.4) | 80 (2.1) | |
| Los Angeles | 501 (9.1) | 159 (9.6) | 342 (8.9) | |
| Rural Georgia | <11 | <11 | <11 | |
| Greater California | 766 (13.9) | 543 (14.2) | 223 (13.4) | |
| Kentucky | 505 (9.2) | 150 (9.0) | 355 (9.3) | |
| Louisiana | 412 (7.5) | 116 (7.0) | 296 (7.7) | |
| New Jersey | 929 (16.9) | 329 (19.8) | 600 (15.7) | |
| Socioeconomic Status | ||||
| Median household income per tract for zip code ($) | 50,204.5±21,195.5 | 52,127.5±22,237.7 | 49,368.7±20,673.5 | <0.001 |
| Percent residents per tract living below poverty level | 8.6±11.1 | 8.3±11.8 | 8.7±10.8 | 0.306 |
| Percent of persons 25+ per tract with <12 years education | 18.5±11.8 | 17.8±11.8 | 18.8±11.8 | 0.003 |
| Follow up (years) | 4.4±2.7 | 4.0±2.4 | 4.6±2.9 | <0.001 |
procedure groups significantly differed by each histologic subtype (clear cell, p<0.001; papillary, p<0.001; chromophobe, p<0.002) with the exception of adenocarcinoma (p=0.256)
To protect patient confidentiality, results with 11 patients or less are reported as N<11 and percentages in the respective columns were rounded in increments of 1%. To avoid derivation of the omitted cells, totals from the next smallest group (*) from each covariate category were similarly altered.
Table II.
Study population demographic and tumor characteristics following propensity adjustment
| Characteristic | Partial Nephrectomy |
Radical Nephrectomy |
P Value |
|---|---|---|---|
| Tumor size (cm) | 2.8±0.8 | 2.8±0.9 | 0.534 |
| Charlson Co-Morbidity Index | 0.87±1.2 | 0.89±1.3 | 0.491 |
| Gender | |||
| Male | 1,551 (56.7) | 1,551 (56.1) | 0.34 |
| Female | 1,183 (43.3) | 1,211 (43.9) | |
| Race/Ethnicity | 0.401 | ||
| Caucasian | 2,308 (84.4) | 2,356 (85.3) | |
| African American | 256 (9.4) | 237 (8.6) | |
| Asian | 51 (1.9) | 50 (1.8) | |
| Hispanic | 62 (2.3) | 61 (2.2) | |
| Other | 58 (2.1) | 58 (2.1) | |
| Marriage Status | 0.258 | ||
| Not married | 1,004 (36.7) | 1,033 (37.4) | |
| Married | 1,730.5 (63.6) | 1,728.5 (62.6) | |
| Area of residence | 0.433 | ||
| Non-metropolitan | 416 (15.2) | 409 (14.8) | |
| Metropolitan | 2,318 (84.8) | 2,352.7 (85.2) | |
| Histologic Type | 0.220 | ||
| Clear cell | 2,256 (82.5) | 2,286 (82.7) | |
| Papillary | 327 (12.0) | 322 (11.6) | |
| Chromophobe* | <150 (<6%) | <150 (<6%) | |
| Adenocarcinoma | <11 | <11 | |
| Grade | 0.27 | ||
| 1 | 520 (19.0) | 498 (18.0) | |
| 2 | 1,146 (41.9) | 1,161 (42.1) | |
| 3 | 339 (12.4) | 343 (12.4) | |
| 4 | 39 (1.4) | 37 (1.4) | |
| missing | 690 (25.2) | 721.5 (26.1) | |
| SEER Region | 0.7219 | ||
| San Francisco | 79 (2.9) | 86 (3.1) | |
| Connecticut | 214 (7.8) | 210 (7.6) | |
| Detroit | 261 (9.6) | 270 (9.8) | |
| Hawaii* | <40 (<2%) | <40 (<2%) | |
| Iowa | 211 (7.7) | 215 (7.8) | |
| New Mexico | 52 (1.9) | 53 (1.9) | |
| Seattle | 151 (5.5) | 154 (5.6) | |
| Utah | 56 (2.1) | 57 (2.1) | |
| Atlanta | 58 (2.1) | 60 (2.2) | |
| San Jose | 59 (2.2) | 53 (1.9) | |
| Los Angeles | 257 (9.4) | 255 (9.2) | |
| Rural Georgia | <11 | <11 | |
| Greater California | 366 (13.4) | 380 (13.8) | |
| Kentucky | 275 (10.1) | 255 (9.3) | |
| Louisiana | 208 (7.6) | 208 (7.5) | |
| New Jersey | 450 (16.5) | 463 (16.8) | |
| Socioeconomic Status | |||
| Median household income per tract for zip code ($) | 49,996±21,379.5 | 50,111.0±21,234.0 | 0.674 |
| Percent residents per tract living below poverty level | 8.8±11.4 | 8.7±11.1 | 0.392 |
| Percent of persons 25+ per tract with <12 years education | 18.7±12.0 | 18.5±11.8 | 0.402 |
To protect patient confidentiality, results with 11 patients or less are reported as N<11 and percentages in the respective columns were rounded in increments of 1%. To avoid derivation of the omitted cells, totals from the next smallest group (*) from each covariate category were similarly altered.
Acknowledgments
This publication was supported in party by grant number P30 CA006927 from the National Cancer Institute (RGU). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. The authors were supported in part through the National Institutes of Health R03CA152388 (BLE), and Department of Defense, Physician Research Training Award (AK).
"This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database."
"The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database."
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2012. CA Cancer J Clin. 2012 doi: 10.3322/caac.20138. in press. [DOI] [PubMed] [Google Scholar]
- 2.Hollingsworth JM, Miller DC, Daignault S, et al. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006;98:1331–1334. doi: 10.1093/jnci/djj362. [DOI] [PubMed] [Google Scholar]
- 3.Smaldone MC, Kutikov A, Egleston BL, et al. Small renal masses progressing to metastases under active surveillance: A systematic review and pooled analysis. Cancer. 2012;118:997–1006. doi: 10.1002/cncr.26369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 5.Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7:735–740. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182:1271–1279. doi: 10.1016/j.juro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Sun M, Trinh QD, Bianchi M, et al. A Non-Cancer-Related Survival Benefit Is Associated With Partial Nephrectomy. Eur Urol. 2011 doi: 10.1016/j.eururo.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 8.Thompson RH, Boorjian SA, Lohse CM, et al. Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol. 2008;179:468–471. doi: 10.1016/j.juro.2007.09.077. discussion 72-3. [DOI] [PubMed] [Google Scholar]
- 9.Zini L, Perrotte P, Capitanio U, et al. Radical versus partial nephrectomy: effect on overall and noncancer mortality. Cancer. 2009;115:1465–1471. doi: 10.1002/cncr.24035. [DOI] [PubMed] [Google Scholar]
- 10.Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol. 2011;59:543–552. doi: 10.1016/j.eururo.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Kutikov A, Smaldone MC, Egleston BL, et al. Should Partial Nephrectomy Be Offered to All Patients Whenever Technically Feasible? Eur Urol. 2011;61:732–734. doi: 10.1016/j.eururo.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Kutikov A, Egleston BL, Wong YN, et al. Evaluating overall survival and competing risks of death in patients with localized renal cell carcinoma using a comprehensive nomogram. J Clin Oncol. 2010;28:311–317. doi: 10.1200/JCO.2009.22.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simhan J, Smaldone MC, Tsai KJ, et al. Objective measures of renal mass anatomic complexity predict rates of major complications following partial nephrectomy. Eur Urol. 2011;60:724–730. doi: 10.1016/j.eururo.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giordano SH, Kuo YF, Duan Z, et al. Limits of observational data in determining outcomes from cancer therapy. Cancer. 2008;112:2456–2466. doi: 10.1002/cncr.23452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 17.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med. 2004;23:2937–2960. doi: 10.1002/sim.1903. [DOI] [PubMed] [Google Scholar]
- 19.Cole SR, Hernan MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75:45–49. doi: 10.1016/j.cmpb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 21.Efron B, Tibshirani RJ. CRC Monographs on Statistics and Applied Probability. Boca Raton, Florida: Chapman and Hall; 1998. An introduction to the bootstrap. [Google Scholar]
- 22.Dulabon LM, Lowrance WT, Russo P, et al. Trends in renal tumor surgery delivery within the United States. Cancer. 2010;116:2316–2321. doi: 10.1002/cncr.24965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hollenbeck BK, Taub DA, Miller DC, et al. National utilization trends of partial nephrectomy for renal cell carcinoma: a case of underutilization? Urology. 2006;67:254–259. doi: 10.1016/j.urology.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 24.Miller DC, Schonlau M, Litwin MS, et al. Renal and cardiovascular morbidity after partial or radical nephrectomy. Cancer. 2008;112:511–520. doi: 10.1002/cncr.23218. [DOI] [PubMed] [Google Scholar]
- 25.Huang WC, Elkin EB, Levey AS, et al. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors--is there a difference in mortality and cardiovascular outcomes? J Urol. 2009;181:55–61. doi: 10.1016/j.juro.2008.09.017. discussion 61-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooperberg MR. Adverse Effects of Androgen Deprivation and the Limits of National Tumor Registries. Eur Urol. 2011 doi: 10.1016/j.eururo.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 27.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182:844–853. doi: 10.1016/j.juro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 28.Ibrahim HN, Foley R, Tan L, et al. Long-term consequences of kidney donation. N Engl J Med. 2009;360:459–469. doi: 10.1056/NEJMoa0804883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dignam JJ. Some additional thoughts on time-varying treatment effects in breast cancer. J Clin Oncol. 2011;29:4469–4470. doi: 10.1200/JCO.2011.37.8166. author reply 70. [DOI] [PubMed] [Google Scholar]
- 30.Jatoi I, Anderson WF, Jeong JH, et al. Breast cancer adjuvant therapy: time to consider its time-dependent effects. J Clin Oncol. 2011;29:2301–2304. doi: 10.1200/JCO.2010.32.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]


