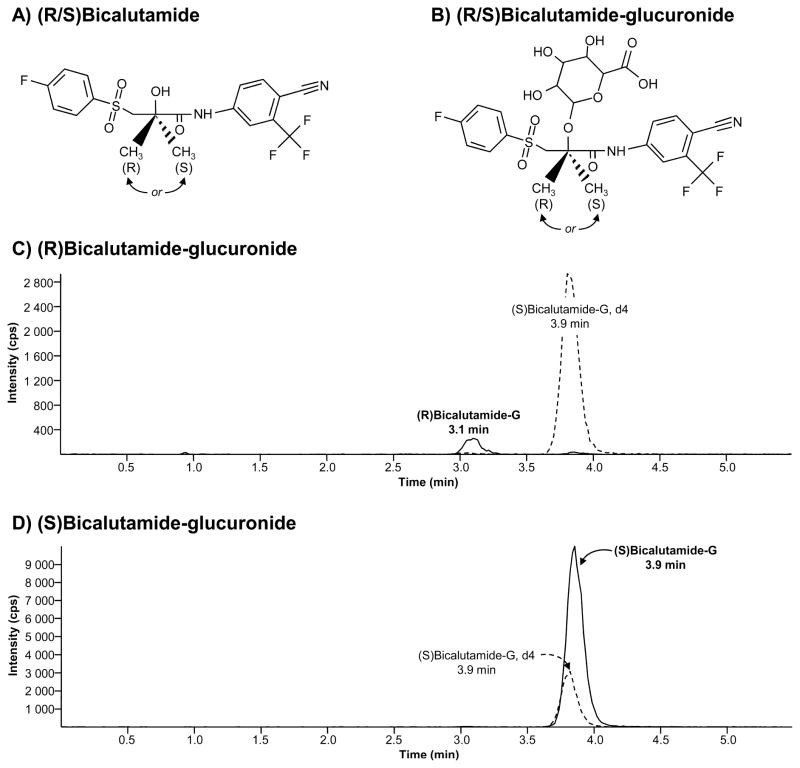
Figure 1. Chemical structure and representative chromatograms of (R) and (S) bicalutamide-glucuronide.
(A and B) Bicalutamide (A) is a pure non-steroidal anti-androgen used in clinic as a racemate of R and S enantiomers. Both enantiomers can be glucuronidated (B).
(C and D) Representative chromatograms of (R)bicalutamide-glucuronide (C) and (S)-glucuronide (D) as obtained from incubated samples. Retention times were 3.1 minutes (min) for (R)bicalutamide-glucuronide and 3.9 minutes for (S)bicalutamide-glucuronide and deuterated internal standard (S)bicalutamide-G, d4.
G: glucuronide; min: minute; cps: count per second