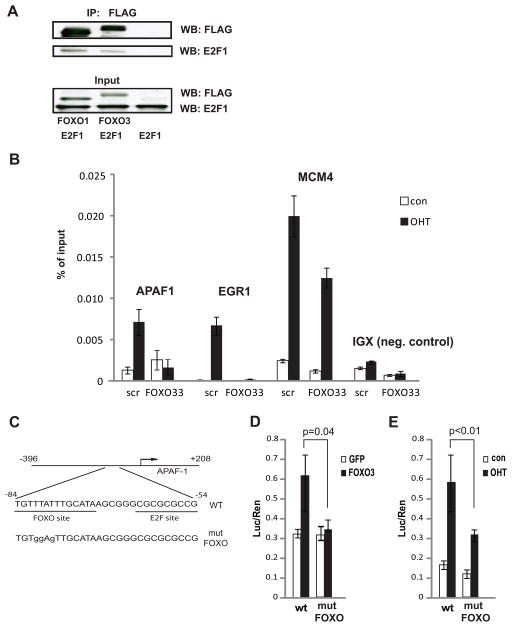
Figure 2. E2F1 and FOXO transcriptional cooperation.
A. 293T cells were cotransfected with the indicated combinations of E2F1 and either FLAG-tagged FOXO1, Flag-tagged FOXO3, or empty vector expression plasmids. Lysates were immunoprecipitated using anti-FLAG antibody and precipitates as well as inputs were analyzed by western blot for the presence of FOXO (using anti-FLAG antibody) and of E2F1 (using anti-E2F1 antibody).
B. U2OS ER-E2F1 cells were infected with lentiviruses encoding an shRNA construct targeting FOXO1 and FOXO3 (FOXO33) or scrambled shRNA (scr). Twenty four hours post infection cells were serum starved for twenty four hours and then medium was replaced to serum-free medium with (OHT) or without (con) 30 nM OHT for four hours.
Chromatin immunoprecipitation was performed with anti-HA antibody to detect binding of ER-HA-E2F1 to the indicated promoters.
C. Schematic representation of APAF1 promoter reporter constructs used in luciferase assays.
D. U2OS cells were co-transfected with APAF1 promoter reporter constructs depicted in (C) as indicated below the graphs together with FOXO3 or GFP control expression constructs. Luciferase assays were performed 48 hours later.
E. U2OS ER-E2F1 cells were transfected with equal amounts of APAF1 promoter reporter constructs depicted in (C) as indicated below the graphs. Twenty four hours post infection cells were serum starved for twenty four hours and then medium was replaced to serum-free medium with (OHT) or without (con) 60 nM OHT. Luciferase assays were performed five hours later.