Abstract
Background and objective
Recent evidence suggests that YKL-40 is a relatively new biomarker of inflammation and it is involved in the pathogenesis of several pulmonary diseases. Details of serum and pleural YKL-40 in pleural effusions however, remain unknown. We aimed to assess whether serum and pleural YKL-40 is an accurate biomarker of pleural effusions.
Methods
This clinical study was prospective, observational and cross-sectional. The concentrations of serum and pleural fluid YKL-40 and conventional pleural marker levels were measured in 80 subjects with pleural effusions, including 23 transudates caused by congestive heart failure (CHF), and 57 exudates including 23 parapneumonic, 22 malignant and 12 tuberculous pleural effusions (TBPEs).
Results
Median pleural fluid YKL-40 levels were higher in exudates than in transudates (219.4 and 205.9 ng/mL, respectively, P<0.001). High pleural YKL-40 levels, with a cutoff value of >215 ng/mL, yielded a 73% sensitivity, 73% specificity, likelihood ratio 2.8 for diagnosing exudate, with an area under the curve of 0.770 [95% confidence intervals (CI): 0.657-0.884]. Pleural YKL-40/serum YKL-40 ratio >1.5 yielded a 75% sensitivity, 72% specificity and likelihood ratio 2.6 for diagnosing TBPE, with an area under the curve of 0.825 (95% CI: 0.710-0.940).
Conclusions
High concentrations of pleural YKL-40 level may help to differentiate exudate from transudate and a high pleural YKL-40/serum YKL-40 ratio may be helpful in seperating TBPE from non-tuberculous effusions.
KEYWORDS : Exudate, pleural effusion, transudate, tuberculosis, YKL-40
Introduction
Chitin is a cellulose (N-acetyl-beta-D-glucosamine) like polysaccaride and found in structural components of arthropodes, insects, nematodes and fungi. Despite lacking of endogenous chitin within human body, the genes which encode chitin and the chitotriosidase enzymes are found in human body (1). The human chitotriosidase has oligosaccaride binding capability and chitinolytic activity toward artificial substrates as well as chitin therefore they called as chitinase. Until now, eight different kinds of chitinases have been discovered together with YKL-40 (2). The three N-terminal aminoacids Tyrosine (Y), Lysine (K) and Leucine (L) and the molecular mass of 40 kDa give the name of YKL-40 (3). Relevant to chitinases, many new researchs have been performed in last two decades to identify the pathogenesis and the prognosis of several diseases including lung pathologies. These studies demonstrated that circulating YKL-40 levels increase in various inflammatory and neoplastic diseases where the tissue damage and remodeling of the extracellular matrix occur (4,5). YKL-40 is released from different kind of cells such as macrophages, neutrophils, mesothelial cells, synovial fibroblasts, smooth muscle cells and malign tumour cells (6). YKL-40 has been shown to be associated with lung injury pathogenesis contributing to inflammation, remodeling and cellular proliferation and a potent growth factor for connective tissue cells (6).
Pleural effusions can be arised from various pathologies and the diagnosis generally needs a systematic evaluation of the patients and a number of diagnostic procedures. Many useful tests have been recommended for evaluation of pleural effusions. Measuring of different biomarkers is also recommended in discriminating parapneumonic, tuberculous and malignant pleural effusions (MPEs). Despite the advances in biochemistry and invasive diagnostic procedures, the etiology of pleural effusions sometimes remains unclear (7). For these reasons, new biomarkers of pleural effusions with high diagnostic accuracies are currently required.
The purpose of this study was to assess the utility of pleural and circulating YKL-40 levels in discriminating the etiology of pleural effusions.
Methods
Study design and patients
This is a prospective, observational, descriptive type and multidisciplinary study which was approved by the ethics committee of Recep Tayyip Erdogan University and was designed in accordance with the Declaration of Helsinki. The study was conducted between 01 December 2012 and 31 May 2013 in the pulmonary department. A total of 80 consecutive subjects with pleural effusions and with the age of >18 years were enrolled in the study. The subjects with history of chronic renal and hepatic disease, any pleural effusions remained undiagnosed after microbiological, biochemical and cytological analysis were excluded from the study. All the subjects gave written informed consent at the time of thoracenthesis.
Laboratory parameters and preparation of the samples
Total blood count, biochemical measurements of serum and pleural effusions were performed at the Department of Clinical Biochemistry Laboratory in Recep Tayyip Erdogan University, using Abbott ci16200 system (Abbott Laboratories, Abbott Park, Illinois, USA) and standard laboratory techniques. The blood samples were drawn at the same time with thoracentesis for the measurement of biochemical parameters and they were collected into different test tubes. Hematologic and biochemical analysis of pleural fluid and serum were performed within hours after the sampling. Pleural adenosine deaminase (ADA) levels were also measured as UI/mL. For serum YKL-40 analysis, the blood samples were centrifuged at 3,000 g for 10 minutes at 4 °C. After the centrifugation, the serum was removed and transfered into a clean test tube. All pleural and serum samples were stored in refrigerator at –80 °C until YKL-40 analysis and they were dissolved and stored 24 hour at 2-8 °C before the study.
Principles of the procedure for YKL-40 measurements
The pleural fluid and serum YKL-40 analysis was performed by an experienced specialist of clinical biochemistry who was blinded to the other clinical diagnosis. YKL-40 protein concentrations were determined in serum and pleural fluids using a spesific kit (MICROVUE YKL-40 EIA Kit, Quidel, San Diego, USA) by an enzyme-binding immunosorbent test (ELISA). Standarts, Controls and the concentration of YKL-40 presented in the test specimens were measured by spectrophotometer (PerkinElmer LAMBDA UV/Vis Spectrophotometer, Massachusetts, USA) and the results are calculated from the generated standard curve using linear regression analysis according to the producer’s instructions (8).
Definitions
Light’s criteria were used to differentiate transudate from exudate (9).
Parapneumonic effusion (PPE)
The presence of pleural effusion in patients with community-acquired pneumonia. We used The American Thoracic Society criteria for defining the pneumonia [newly occured respiratory symptoms, fever and abnormal breath sounds, together with a new pulmonary infiltration on chest X-ray, and consistent laboratory findings (leukocytosis count and high serum CRP levels) and neutrophilic pleural effusion (10)].
MPE
Active neoplastic disease in any location and malignancy on pleural cytological or histological examination.
Tuberculous pleural effusion (TBPE)
Pleural biopsy specimen showing granulomas with caseification necrosis, or pleural fluid positive on Ziehl-Nielsen stain or Lowenstein-Jensen culture, or pleural fluid consistent with clinical signs and symptoms of tuberculosis together with lymphocyte predominance in pleural effusion and good response to anti-tuberculosis treatment.
Congestive heart failure (CHF)
The diagnosis of CHF was made according to clinical symptoms, including history, chest radiography, response to diuretic therapy and a left ventricular ejection fraction of ≤40% measured by echocardiography.
Statistical analysis
All statistical evaluations were performed with the Statistical Package for Social Sciences (SPSS), version 21 (SPSS Inc, Chicago, IL, USA). Variables were presented as the mean ± standard deviation, and nonparametric variables were shown as median, min-max values and inter-quartile range from the 25th to the 75th percentiles. The Kolmogorov-Smirnov test was used to analyse the normal distribution of the variables. The ANOVA with post hoc analysis (Tamhane’s T2) has been used for the normally distributed data, Kruskal Wallis and Mann-Whitney U test has been used for non-normal data. Correlations between quantitative data were analyzed by Spearman’s correlation test. Receiver-operating characteristic analysis assessed the optimal cut-off values of pleural fluid YKL-40 levels for exudates, TBPE, PPE and MPE. Diagnostic performances of the biomarker by using sensitivity, specificity, positive predictive value, negative predictive value, accuracy and likelihood ratios with their respective 95% confidence intervals (CI) were calculated according to standard formulae. P value <0.05 was considered to be significant.
Results
The study group consisted of 80 subjects (56 men, 24 women, mean age 67±17 years) with pleural effusions, including 23 (28.8%) transudates caused by CHF, and 57 (71.2%) exudates. There were 23 (28.8%) PPE, 22 (27.5%) MPE and 12 (15%) TBPE among the exudative effusions. The mean ages were 75±11 years in CHF, 70±11 years in MPE, 69±13 years in PPE, 40±15 years in TBPE.
Primary tumours in MPE cases were mostly lung cancers (n=18, 82%) and miscellaneous organ tumors (n=4, 18%) (one gastric cancer, one prostate cancer, one breast cancer, one hepatocellular carcinoma).
The associations between the serum YKL-40 and pleural YKL-40 levels, and the results were analyzed by Pearson correlation analysis. The details of the results were shown in Table 1. In this study we observed that the serum YKL-40 levels were associated with age and serum CRP level and showed negative correlations with serum protein, serum albumin, pleural albumin level, pleural leucocyte number and pleural lymphocyte ratio. Pleural YKL-40 levels correlated with serum CRP, serum YKL-40 levels, blood lymphocytes, pleural lymphocytes, protein and albumin, pleural LDH, pleural triglyceride, pleural cholesterol. Pleural YKL-40 showed a negative correlation with pleural glucose level.
Table 1. The correlation analysis of serum and pleural YKL-40 levels (ng/mL) according to blood and pleural parameters.
| Characteristics | Serum YKL-40, ng/mL | Pleural YKL-40, ng/mL | |||
|---|---|---|---|---|---|
| r value | P value | r value | P value | ||
| Age, year | 0.477 | <0.001* | –0.112 | 0.325 | |
| Blood | |||||
| Leucocyte, ×103/mL | 0.244 | 0.029 | 0.171 | 0.130 | |
| Neutrophils, % | 0.205 | 0.068 | 0.048 | 0.673 | |
| Lymphocytes, % | –0.023 | 0.843 | 0.254 | 0.023* | |
| Glucose, mg/dL | 0.138 | 0.233 | 0.022 | 0.848 | |
| Protein, g/dL | –0.226 | 0.044* | 0.144 | 0.202 | |
| Albumin, g/dL | –0.555 | <0.001* | –0.013 | 0.911 | |
| LDH, U/L | 0.182 | 0.105 | 0.540 | 0.634 | |
| Triglyceride, mg/dL | –0.026 | 0.820 | 0.213 | 0.057 | |
| Cholesterol, mg/dL | –0.057 | 0.616 | 0.189 | 0.093 | |
| Creatinine, mg/dL | 0.199 | 0.077 | –0.021 | 0.853 | |
| CRP, mg/L | 0.320 | 0.004* | 0.272 | 0.015* | |
| YKL-40, ng/mL | 0.319 | 0.004* | |||
| Pleural effusion | |||||
| Glucose, mg/dL | 0.039 | 0.732 | –0.232 | 0.038* | |
| Protein, g/dL | –0.152 | 0.177 | 0.529 | <.001* | |
| Albumin, g/dL | –0.379 | 0.001* | 0.433 | 0.001* | |
| LDH, U/L | 0.000 | 1 | 0.375 | 0.001* | |
| Triglyseride, mg/dL | –0.001 | 0.992 | 0.388 | <0.001* | |
| Cholesterol, mg/dL | –0.195 | 0.084 | 0.525 | <0.001* | |
| ADA, UI/mL | 0.074 | 0.512 | –0.027 | 0.814 | |
| Leucocyte, ×103/mL | –0.313 | 0.005* | 0.028 | 0.805 | |
| Neutrophils, % | –0.156 | 0.250 | 0.048 | 0.725 | |
| Lymphocytes, % | 0.098 | 0.478 | 0.382 | 0.006* | |
*, P values <0.05 were accepted as significant, Spearman’s correlation test was used for analysis; ADA, adenosine deaminase.
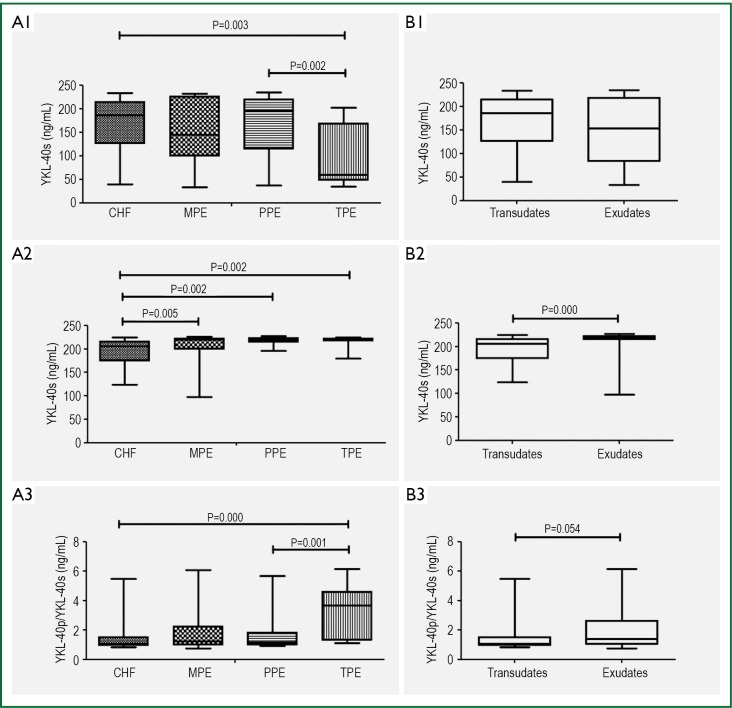
The serum YKL-40, pleural YKL-40 and the ratio of plevral YKL-40 to serum YKL-40 were measured and the minimum values, the 25th, 50th and 75th percentiles, and the maximum values of the study groups were compared and shown in Figure 1. We found a higher level of serum YKL-40 and serum CRP in exudates compared to transudates (Table 2).
Figure 1.
Box plots showing serum YKL-40 (YKL-40s) levels (A1), pleural YKL-40 levels (YKL-40p) (A2) and the ratio of pleural YKL-40 and serum YKL-40 levels (A3) among the subjects with congestive heart failure (CHF), tuberculous pleural effusions (TBPE), parapneumonic effusions (PPE), and malignant pleural effusions (MPE). Similarly, B1, B2 and B3 show the box plots of YKL-40s, YKL-40p and the ratio of YKL-40p/YKL-40s among transudates and exudates, respectively. The box plots show the minimum value, the 25th, 50th and 75th percentiles, and the maximum value. Specific data are detailed in Tables 2,3.
Table 2. Comparison of the study groups with transudates and exudates in the aspect of serum YKL-40, pleural YKL-40 and serum CRP levels.
| Parameters | Transudate (n=23) | Exudate (n=57) | P |
|---|---|---|---|
| Serum YKL-40, ng/mL | 186.1 (39-233) | 153.2 (33-234) | 0.422 |
| Pleural YKL-40, ng/mL | 205.9 (123-224) | 219.4 (97-227) | <0.001* |
| YKL-40pl / YKL-40s ratio | 1.04 (0.82-5.46) | 1.39 (0,76-6,14) | 0.054 |
| CRP, mg/L | 3 (0,18-12,3) | 9 (0,02-38) | 0.001* |
Serum YKL-40, pleural YKL-40 and serum CRP levels did not distributed normally between the patient groups with exudates and transudates, the results were shown as median (min, max), The Kruskal-Wallis test and Mann-Whitney U test after Bonferroni adjustments were used for the nonparametric variables, transudates included the patients with congestive heart failure and exudates included the patients with malignant effusion, parapneumonic effusion and tuberculous effusion, *, P values <0.05 were accepted as significant; pl, pleural; s, serum.
We compared the serum YKL-40, pleural YKL-40 and serum CRP levels between the study groups (Table 3). Pleural YKL-40 was found lower in the subjects with CHF. Serum YKL-40 levels were found lower in TBPE group compared to others and the ratio of plevral YKL-40 to serum YKL-40 was found higher in TBPE group compared to other groups (P<0.001). As an expected result, serum CRP levels were higher in PPE group than the others in present study (P<0.001).
Table 3. Comparison of the study groups in the aspect of serum and pleural YKL-40 and serum C-reactive protein levels.
| Parameters | CHF (n=23) | MPE (n=22) | PPE (n=23) | TPE (n=12) |
|---|---|---|---|---|
| †Serum YKL-40, ng/mL | 186.1 [39-233] | 162.9 [33-232] | 195.8 [37-234] | 65.15 [35-202]* |
| †Pleural YKL-40, ng/mL | 205.9 [123-224]* | 219.6 [155-226] | 219 [97-227] | 219.3 [179-225] |
| †YKL-40pl/YKL-40s ratio | 1.04 [0.82-5.46] | 1.3 [0.95-6.07] | 1.07[0.76-5.66] | 3.38 [1.1-6.14]* |
| ‡CRP, mg/L | 4.15±3.46 | 6.11±4.04 | 15.04±10.27* | 9.15±7.67 |
| †Pleural ADA, UI/mL | 13 [1-23] | 13 [1-35] | 15 [1-48] | 45 [20-97]* |
CHF, Congestive heart failure; MPE, Malignant pleural effusion; PPE, Parapneumonic effusion; TPE, Tuberculous pleural effusion; *, P values were found <0.05 between the patient groups; †, Serum YKL-40, pleural YKL-40 and pleural ADA levels did not distributed normally between the groups, so the results were shown as median and (min-max), The Kruskal-Wallis test and Mann-Whitney U test after Bonferroni adjustments were used for the nonparametric variables; ‡, Serum C-reactive protein (CRP) levels distributed normally and the results were shown as mean and standart deviation, ANOVA were used for the comparison of averages for the parametric variables; pl, pleural; s, serum; ADA, adenosine deaminase.
Pleural YKL-40/serum YKL-40 ratios were found high in tuberculous effusions and different between TBPE and non-TBPE (P<0.001). Serum YKL-40 and pleural YKL-40 were not different between MPE and non-MPEs, and similarly these parameters were not different between PPE and other exudative pleural effusions (P>0.05).
Pleural effusion YKL-40 levels had the highest diagnostic accuracy for identifying exudates as measured by the area under receiver-operating characteristic curve analysis (area under the curve of 0.770) (Figure 2). The sensitivity, specificity, likelihood ratio of pleural YKL-40 for diagnosing exudate at the optimal cut-off level of 215 ng/mL was 73%, 73%, and 2.8 respectively. The positive predictive value of pleural YKL-40 for exudate was found to be high (87%) (Table 4). Pleural YKL-40/serum YKL-40 ratio >1.5 yielded 75% sensitivity, 72% specificity and likelihood ratio 2.6 for diagnosing TBPE, with an area under the curve of 0.825 (95% CI: 0.710-0.940) (Figure 3).
Figure 2.
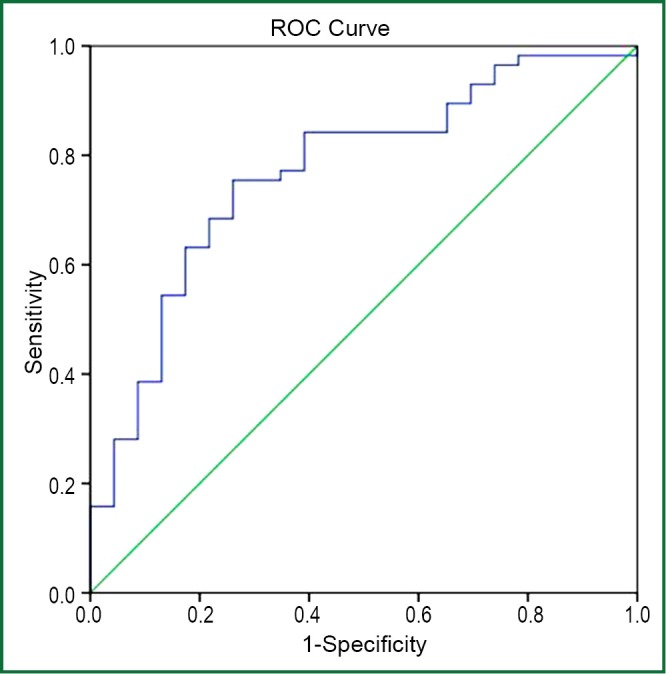
Receiver-operating characteristic (ROC) curves of pleural YKL-40 (>215 ng/mL) in differentiating exudates from transudates. Sensitivity: 73%, specificity: 73% and AUC (area under the curve): 0.770 (95% CI: 0.657-0.884).
Table 4. Diagnostic accuracy of pleural YKL-40 and pleural YKL-40/serum YKL-40 ratio in pleural effusions.
| Diagnosis | Parameter | AUC | Cutoff value | Sensitivity | Specificity | PPV | NPV | LR | Accuracy |
|---|---|---|---|---|---|---|---|---|---|
| Exudate | Pleural YKL-40 | 0.770 (0.657-0.884) | 215 | 0.73 | 0.73 | 0.87 | 0.53 | 2.8 | 0.73 |
| YKL-40pl/YKL-40s | 0.638 (0.510-0.767) | 1.2 | 0.57 | 0.65 | 0.80 | 0.38 | 1.6 | 0.60 | |
| TPE | Pleural YKL-40 | 0.632 (0.488-0.777) | 217 | 0.75 | 0.52 | 0.22 | 0.92 | 1.5 | 0.56 |
| YKL-40pl/ YKL-40s | 0.825 (0.710-0.940) | 1.5 | 0.75 | 0.72 | 0.32 | 0.94 | 2.6 | 0.72 | |
| MPE | Pleural YKL-40 | 0.580 (0.442-0.718) | 218 | 0.54 | 0.58 | 0.33 | 0.77 | 1.3 | 0.57 |
| YKL-40pl/ YKL-40s | 0.520 (0.379-0.662) | 1.2 | 0.54 | 0.51 | 0.30 | 0.75 | 1.1 | 0.52 | |
| PPE | Pleural YKL-40 | 0.610 (0.471-0.749) | 218 | 0.56 | 0.57 | 0.35 | 0.76 | 1.3 | 0.57 |
| YKL-40pl/ YKL-40s | 0.584 (0.448-0.719) | 1.2 | 0.56 | 0.56 | 0.34 | 0.76 | 1.2 | 0.56 |
TPE, tuberculous pleural effusion; MPE, malignant pleural effusion; PPE, parapneumonic effusion; AUC, area under curve; PPV, positive predictive value; NPV, negative predictive value; LR, likelihood ratio.
Figure 3.
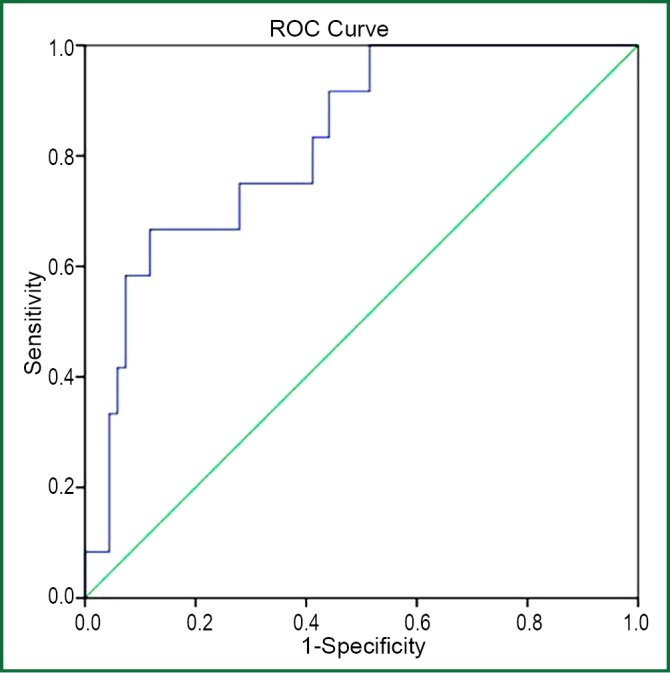
Receiver-operating characteristic (ROC) curves of pleural YKL-40/serum YKL-40 ratio (>1.5) in differentiating tuberculous from non-tuberculous pleural effusions. Sensitivity: 75%, specificity: 72% and AUC (area under the curve): 0.825 (95% CI: 0.710-0.940).
The results of pleural ADA levels were found to be high in pleural tuberculosis compared to other pleural fluids (P<0.001).
Discussion
The most remarkable results of this study are that high pleural YKL-40 levels are sensitive in discriminating exudates from transudates, and also TBPE from non tuberculous effusions. High pleural YKL-40/serum YKL-40 ratios yielded highest sensitivity in discriminating TBPE from non-TBPE.
In recent years, many markers have been investigated for the differential diagnosis of pleural effusions. Inflammatory markers such as CRP, interleukin-6, tumor necrosis factor-α elevates in PPE according to MPE (11). Similar the results of the study of Daniil et al. (11) we found a higher serum CRP levels in exudates than transudates and also in PPE than the other subtypes of pleural effusions in the present study. In another study Lin et al. (12) showed higher pleural fluid levels of procalcitonin as an inflammatory marker in PPE than in the others. In another study, Chen et al. (13) reported different results from these mentioned above, as pleural CRP levels findings were the same in the PPE subtypes. Ozsu et al. (14) investigated a novel biomarker in pleural effusions and they showed higher pleural fluid pentraxin-3 levels in PPE than in other causes of exudates. These studies showed that biomarkers of inflammation are useful in discriminating parapneumonic from the other pleural effusions.
YKL-40 is a relatively new biomarker of inflammation and it has been furtherly investigated in several pathologic conditions. Funkhouser et al. (15) reported that the levels of YKL-40 in the lung and serum are increased in asthma and other inflammatory and remodeling disorders correlating with disease severity. Bouzas et al. (16) found that the chitotriosidase activity as a chitinase enzyme was significantly higher in TBPE than in the non-tuberculous lymphocytic pleural effusions (P<0.01) with a sensitivity of 92% and specificity of 72%. Kim et al. (17) studied YKL-40 levels in different lung diseases and pleural effusions, they found that the results of serum YKL-40 were the highest in tuberculosis compared with other pleural effusions including parapneumonic, malignant, and cardiogenic effusions. In the present study pleural YKL-40 levels yielded a diagnostic value in pleural effusions and we found a positive correlation between pleural YKL-40 levels and serum CRP, serum YKL-40 and pleural levels of LDH, protein, cholesterol and triglyseride levels. Our study results also revealed that serum and pleural YKL-40 did not help to differentiate subgroups of exudates except tuberculous effusions with a high rate of pleural to serum YKL-40 level. The subjects with tuberculosis who enrolled in this study were relatively young individuals. Serum YKL-40 levels show a positive correlation with age, as we found in this study, so the existence of relatively lower levels of serum YKL-40 in patients with TBPE may be related to be younger age.
Pleural YKL-40 levels showed a positive correlation with serum CRP and YKL-40 levels, and pleural levels of albumin, LDH, triglyceride and cholesterol. These associations are thought that the pleural diseases accompanying with exudates such as infections and malignant disease may cause an elevation in serum and pleural YKL-40 levels.
Vanham et al. showed that the CD14+, CD16+ monocytes are increased in numbers in patients with tuberculosis (18). These monocytes are believed to be a more mature version of monocytes with properties of tissue macrophages, probably of proinflammatory type. They have a similar antigen-presenting potential as macrophages and produce proinflammatory cytokines, but produce little or no anti-inflammatory cytokines and YKL-40 mRNA expression is found in these cells (19).
YKL-40 is a member of chitotriosidases and in previous studies pleural chitotriosidase level was found to be higher in TBPE than nonspecific pleural effusion. Besides, by Bauzas et al. (16) demonstrated a significant relation between chitotriosidase and ADA activities, although we did not find such a correlation between serum and pleural YKL-40 and ADA levels. Contrary to our results, Kim et al. (17) reported that serum YKL-40 level of the patients with pleural tuberculous was higher than that of the patients with effusions due to malignant or cardiac causes.
We found that the diagnostic performance of serum and pleural YKL-40 were not apparently superior to that of CRP in discriminating parapneumonic from other exudative pleural effusions and to that of pleural ADA in discriminating tuberculous PE from non-tuberculous PE. In the view of the present results, we conclude that YKL-40 is released from different cells types in the pleural space and did not reflect local inflammation better than CRP and did not reflect tuberculosis better than ADA. But these results showed that it is not enough to say that the measurement of serum and pleural YKL-40 level is practical in clinical use with these limited diagnostic rates.
There are some limitations in this study. The relatively small sample size of the study group may have restricted the statistical power. In addition, the correlation analysis between serial pleural YKL-40 levels and the treatment responses could not be evaluated. Consequently it is needed to further studies consisting of large populations and different age groups with respect to the diagnostic values of YKL-40 levels.
In conclusion, high concentrations of YKL-40 in pleural effusions may help to differentiate exudate from transudates and high pleural YKL-40/serum YKL-40 ratios may be helpful in seperating TBPE from non-tuberculous effusions but these tests are inadequate for discriminating the reasons of pleural effusions alone.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Renkema GH, Boot RG, Muijsers AO, et al. Purification and characterization of human chitotriosidase, a novel member of the chitinase family of proteins. J Biol Chem 1995;270:2198-202 [DOI] [PubMed] [Google Scholar]
- 2.Duru S, Yüceege M, Ardıç S.Chitinases and lung diseases. Tuberk Toraks 2013;61:71-5 [DOI] [PubMed] [Google Scholar]
- 3.Johansen JS, Williamson MK, Rice JS, et al. Identification of proteins secreted by human osteoblastic cells in culture. J Bone Miner Res 1992;7:501-12 [DOI] [PubMed] [Google Scholar]
- 4.Johansen JS. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan Med Bull 2006;53:172-209 [PubMed] [Google Scholar]
- 5.Lee CG, Da Silva CA, Dela Cruz CS, et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol 2011;73:479-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kzhyshkowska J, Gratchev A, Goerdt S.Human chitinases and chitinase-like proteins as indicators for inflammation and cancer. Biomark Insights 2007;2:128-46 [PMC free article] [PubMed] [Google Scholar]
- 7.Alemán C, Sanchez L, Alegre J, et al. Differentiating between malignant and idiopathic pleural effusions: the value of diagnostic procedures. QJM 2007;100:351-9 [DOI] [PubMed] [Google Scholar]
- 8.Harvey S, Weisman M, O’Dell J, et al. Chondrex: new marker of joint disease. Clin Chem 1998;44:509-16 [PubMed] [Google Scholar]
- 9.Light RW, Macgregor MI, Luchsinger PC, et al. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972;77:507-13 [DOI] [PubMed] [Google Scholar]
- 10.Niederman MS, Mandell LA, Anzueto A, et al. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med 2001;163:1730-54 [DOI] [PubMed] [Google Scholar]
- 11.Daniil ZD, Zintzaras E, Kiropoulos T, et al. Discrimination of exudative pleural effusions based on multiple biological parameters. Eur Respir J 2007;30:957-64 [DOI] [PubMed] [Google Scholar]
- 12.Lin MC, Chen YC, Wu JT, et al. Diagnostic and prognostic values of pleural fluid procalcitonin in parapneumonic pleural effusions. Chest 2009;136:205-11 [DOI] [PubMed] [Google Scholar]
- 13.Chen SC, Chen W, Hsu WH, et al. Role of pleural fluid C-reactive protein concentration in discriminating uncomplicated parapneumonic pleural effusions from complicated parapneumonic effusion and empyema. Lung 2006;184:141-5 [DOI] [PubMed] [Google Scholar]
- 14.Ozsu S, Abul Y, Mentese A, et al. Pentraxin-3: A novel biomarker for discriminating parapneumonic from other exudative effusions. Respirology 2013;18:657-62 [DOI] [PubMed] [Google Scholar]
- 15.Funkhouser JD, Aronson NN., Jr Chitinase family GH18: evolutionary insights from the genomic history of a diverse protein family. BMC Evol Biol 2007;7:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouzas L, San José E, Tutor JC. Chitotriosidase activity in pleural effusions. Clin Lab 2007;53:449-52 [PubMed] [Google Scholar]
- 17.Kim HR, Jun CD, Lee KS, et al. Levels of YKL-40 in pleural effusions and blood from patients with pulmonary or pleural disease. Cytokine 2012;58:336-43 [DOI] [PubMed] [Google Scholar]
- 18.Vanham G, Edmonds K, Qing L, et al. Generalized immune activation in pulmonary tuberculosis: co-activation with HIV infection. Clin Exp Immunol 1996;103:30-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johansen JS. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan Med Bull 2006;53:172-209 [PubMed] [Google Scholar]